| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 54, 2018
|
|
|---|---|---|
| Article Number | 13 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/limn/2018005 | |
| Published online | 05 April 2018 | |
Research Article
Asplanchna-kairomone induces life history shifts in Brachionus angularis (Rotifera)
Provincial Key Laboratory for Conservation and Utilization of Important Biological Resource in Anhui, College of Life Sciences, Anhui Normal University,
Wuhu
241000,
Anhui Province, PR China
* Corresponding author: ylxi1965@126.com
Received:
28
October
2017
Accepted:
29
January
2018
Predator-derived kairomones play an important role in ecological and evolutionary processes that enable the prey to survive predation pressure. In the presence of predatory Asplanchna, some Brachionus and Keratella species develop morphological and behavioral defenses, but whether rapid population growth and diapause are inducible defenses largely remains unknown. In the present study, parental B. angularis females cultured at 1.0 × 106 and 2.0 × 106 cells · mL−1 of Scenedesmus obliquus were indirectly exposed to 0, 40, 80 and 160 ind. L−1 of A. brightwelli using mesh enclosure, and their life-table demographic parameters, population growth rates and morphological characters were calculated and measured. The results showed that Asplanchna-released kairomone decreased significantly average lifespan, life expectancy at hatching, generation time and net reproduction rate, but increased the proportion of sexual offspring of parental B. angularis females. The threshold Asplanchna density required for significant effects varied with food level. Kairomone released by Asplanchna at 80 ind. L−1 increased significantly the intrinsic rate of population increase of B. angularis cultured at 2.0 × 106 cells · mL−1 of S. obliquus, which would offset the mortality of exposed females from predation. The accumulation of kairomone in aquatic environments enhanced the indirect effect of Asplanchna on the population growth of B. angularis. The present results indicated that rapid population growth of B. angularis induced by Asplanchna kairomone might facilitate the coexistence of preys with predators, and higher proportion of sexual offspring and then resting egg production might help the preys avoid the predator in time instead of facing the enemy through defenses.
Key words: rotifer / predation / life table demography / population growth rate / sexual reproduction / morphometrics
© EDP Sciences, 2018
1 Introduction
Predation is one of the most important factor structuring zooplankton communities (Williams, 1997). In the presence of predators, many planktonic invertebrates develop various inducible defenses that reduce the risk of predation. Defenses induced by various invertebrate or vertebrate predators have been extensively studied in Daphnia Straus and several genera of rotifers (reviewed in Larsson and Dodson, 1993; De Meester et al., 1999; Gilbert, 1999; Tollrian and Dodson, 1999; Lass and Spaak, 2003). In Daphnia, the defenses may be changes in morphology (e.g. neckteeth formation, tail spine elongation, helmet enlargement), behavior (e.g. diel vertical migration, enhanced capability to escape, swarming) or life history (e.g. age or size at first reproduction, reproductive rate, diapause induction) (reviewed in Lass and Spaak, 2003; reviewed in Gilbert, 2013). In rotifers, the known predator-induced defenses are morphological (e.g. Soto and Sarma, 2009; Yin et al., 2017; reviewed in Gilbert, 2017) and behavioral (Peña-Aguado et al., 2008; Gilbert, 2014), but whether the life history shifts such as rapid population growth and diapause are inducible defenses largely remains unknown.
Brachionus angularis Gosse is a common rotifer species in many natural waterbodies. Different from some species of Brachionus Pallas and Keratella Bory de St. Vincent, B. angularis does not develop any remarkably elongated spines in response to Asplanchna-released kairomone. As another undefended rotifer species, Synchaeta pectinata Ehrenberg was speculated to develop a high population growth rate that offsets mortality from predation (Wallace et al., 2006), but the experimental evidence is scarce. If undefended S. pectinata can develop a high population growth rate, B. angularis should also be able to develop a high population growth rate in response to Asplanchna-released kairomone. If so, what is the Asplanchna density resulting in the high population growth rate in this species?
In cladocerans, diapause has been proposed as a predator avoidance strategy (Hairston, 1987). Kairomones released by fish predators induced directly diapause in a population of D. magna Strauss (Ślusarczyk, 1995, 1999, 2001; Pijanowska and Stolpe, 1996). In rotifers, kairomone in the medium conditioned by A. brightwellii Gosse at 10 and 100 ind. L−1 decreased the proportion of sexual offspring of B. calyciflorus (Yin et al., 2015), but that conditioned by A. brightwellii at 100 ind. L−1 did not affect the proportion of sexual offspring of B. angularis (Yin et al., 2017). Because the Asplanchna kairomone is very unstable (Gilbert, 1967; Halbach, 1970) and thus the use of Asplanchna-conditioned media is also problematic (reviewed in Gilbert, 2013), the effect of kairomone released directly by Asplanchna on the proportion of sexual offspring in rotifers needs further investigation.
The present study investigated the effect of kairomone released by different densities of A. brightwellii on life-table demographic parameters, population growth rates and the morphological characters of both lorica and egg of parental B. angularis females cultured at two algal densities, with the aim of testing the following three hypotheses: (i) B. angularis develops a high population growth rate in response to kairomone released by a certain density of Asplanchna, based on the speculation on S. pectinata (Wallace et al., 2006); (ii) kairomone released by a certain density of Asplanchna shortens the generation time and thus increases the population growth rate because it decreases the reproduction rate of B. angularis (Yin et al., 2017); and (iii) similar to the effect of fish kairomone on D. magna (Ślusarczyk, 1995, 1999, 2001; Pijanowska and Stolpe, 1996), higher concentrations of Asplanchna kairomone induce higher levels of sexual reproduction in B. angularis.
2 Materials and methods
2.1 Sample collection and culture
Individuals of A. brightwellii and B. angularis were collected from Lake Jinghu (31°36′11″N, 118°38′23″E) in July and September 2016, respectively, identified morphologically under a microscope, and clonally cultured in rotifer culture medium (Gilbert, 1963) at 20 ± 1 °C. B. angularis was fed 1.0 × 106 cells · mL−1 of Scenedesmus obliquus (Turp.) Kütz which was semi-continuously cultured in HB-4 medium (Li et al., 1959). Algal cells at the exponential phase of growth were harvested by centrifugation at 3,000 rpm for 5 min, resuspended in rotifer culture medium and stored at 4 °C. The density of algal cells was determined by counting using a haemocytometer. A. brightwellii was maintained at a density of 5000 ind. L−1 in 500 mL beakers and daily fed 30 000 ind. L−1 of B. angularis. All clones of A. brightwellii and B. angularis were cultured in the laboratory for at least 6 months, and one clone of each species was randomly selected for the experiments. For mass cultures of the rotifers and all the experiments, an illumination incubator with a 16:8-h light: dark photoperiod at 130 lx at 20 ± 1 °C was used.
2.2 Life table experiments
Life table experiments of B. angularis subject to Asplanchna-released kairomone were conducted in beakers each containing 50 mL of culture medium. Prior to the life table experiments, the B. angularis clone was maintained at the designated food levels for more than 5 days to allow acclimation. To separate predators from preys but allow the diffusion of Asplanchna kairomone, a small mesh container which was prepared by covering a 25 µm pore-sized mesh at the downside end of a pipe (length: 10 cm, diameter: 1.5 cm) was hung in water column of each beaker with the upside end above the water surface. The water volume in each pipe was adjusted to 10 mL by moving the pipe up or down. For the life-table experiments, chosen number of A. brightwellii individuals (<12 h old) was introduced in each mesh container and 20 neonates (<12 h old) of B. angularis from pre-cultures were added in each beaker. To maintain the activity of A. brightwellii, 10 individuals of B. angularis was added into each mesh container (Dumont and Sarma, 1995). Based on the ranges of A. brightwellii density in subtropical shallow lakes such as Lake Tingtang (Xie et al., 2015; Zhang et al., 2017) and S. obliquus density suitable for the population growth of B. angularis (Peng et al., 2016), we chose four predator densities (0, 40, 80 and 160 ind. L−1) and two algal food levels (1.0 × 106 and 2.0 × 106 cells · mL−1), with three replicates for each treatment. Following initiation of the experiments, every 12 h the original cohort and neonates produced in each beaker were counted; the former were returned to the medium, and the latter were removed into a new beaker and cultured to calculate proportion of sexual offspring. Every 24 h, the surviving original B. angularis individuals were transferred into a fresh beaker containing 50 mL of medium with the designed level of S. obliquus, and the predators in each mesh container were replaced by a new batch with the same age and density, thus the predation pressure could be consistent throughout the experiments. Experiments were terminated when all mother rotifers died.
The age-specific survivorship (lx) and fecundity (mx), life expectancy at hatching (e0), average lifespan (LS), generation time (T), net reproduction rate (R0), intrinsic rate of population increase (rm) and proportion of sexual offspring (PS) were calculated using the following formulae (Pianka, 1988):
Net reproductive rate: 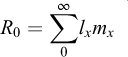
Generation time: 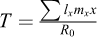
Intrinsic rate of population increase (r), first an approximation using: r − rough = lnR0/T
For final calculation, we solved the equation: 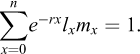
2.3 Population growth experiments
The experiment design, including the predator density, the algal food level and the culture volume, was the same as the life-table experiments. 100 neonates (<12 h old) of B. angularis from pre-cultures were initially introduced in each beaker, and the populations were allowed to grow for 3 days (Xi et al., 2007). Thereafter, the number of B. angularis individuals in each beaker was counted, and the rate of population growth (r) was calculated using the exponential equation (Poole, 1974):
r = (lnNt − lnN0)/t
where N0 and Nt are the initial and final population densities (ind. L−1) of B. angularis, respectively, and t is the time in days.
2.4 Measurement of morphological parameters
After the 3-day population growth, 25–30 B. angularis individuals bearing one or two amictic eggs were sampled from each treatment. After washing in distilled water for 30 min, these animals were fixed in 4% formaldehyde solution. The length and width of lorica, and the long and short diameter of egg of each animal were measured under a microscope using the MC-D500U(E) Digital Camera (Phenix, Jiangxi, China) at 200 × magnification. Thereafter, body size (Vb) was calculated by Vb = 0.2a2b, where a and b represents the lorica length and width, respectively. Egg volume (Ve) was calculated by Ve = 3π(a2b + ab2)/4, where a and b is the long and short diameter of egg, respectively (Zhang and Huang, 1991).
2.5 Statistical analyses
All statistical analyses were performed using SPSS 11.5. The Levene's test was performed to test the homogeneity of variances. Kaplan-Meier analyses were conducted to test for the differences in the survivorships of the rotifer cohorts among the four Asplanchna-released kairomone concentrations. Two-way ANOVA was conducted to analyze the significant effects of algal level, Asplanchna-released kairomone concentration and their interactions on each parameter, and multiple comparisons of LSD were performed to determine which groups were significantly different among the four Asplanchna- released kairomone concentrations at a specific food level. Paired t-tests were carried out to identify the differences of each variable between two algal densities. Results with P values of less than 0.05 were considered statistically significant.
3 Results
3.1 Life table demography
The age-specific survivorship of B. angularis was not affected by Asplanchna-released kairomone concentration (P > 0.05). Of course, limited samples (N = 3) may lead the statistical power to detect significant differences is quite low. The age-specific fecundity curves of B. angularis exposed to Asplanchna-released kairomone generally showed the hump-shaped sawtooth-like pattern. Compared to the controls, and at 1.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 80 and 160 ind. L−1 decreased the peak fecundities of B. angularis by 40.3% and 48.9%, respectively (P < 0.05). At 2.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 40–160 ind. L−1 decreased the peak fecundities of B. angularis by 33.5%, 44.7% and 42.1%, respectively (P < 0.05) (Fig. 1).
The main life-table demographic parameters of B. angularis in relation to Asplanchna-released kairomone concentration and food level are presented in Figure 2. At both algal levels, Asplanchna- released kairomone concentration affected significantly nearly all the life table demographic parameters (P < 0.05) except the intrinsic rate of population increase of B. angularis cultured at 1.0 × 106 cells · mL−1 of S. obliquus (P>0.05). Compared with the controls, and at 1.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 40–160 ind. L−1 shortened significantly the life expectancy at hatching and the average lifespan by 8.5%, 12.8% and 8.5%, and 9.5%, 14.3% and 9.5%, respectively. Kairomone released by Asplanchna at 80–160 ind. L−1 decreased the generation time and the net reproduction rate by 21.2% and 18.2%, and 33.9% and 40.7%, but increased the proportion of sexual offspring by 126.5% and 191.2%, respectively. (Fig. 2).
The increase in algal density affected the magnitudes of effects of Asplanchna-released kairomone concentration on the life table demographic parameters. Compared with the controls, and at 2.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 80–160 ind. L−1 shortened significantly the life expectancy at hatching and the average lifespan by 13.6% and 20.5%, and 15.4% and 23.1%, respectively. Kairomone released by Asplanchna at 40–160 ind. L−1 decreased the generation time and the net reproduction rate by 18.8% and 9.4%, and 35.8% and 45.3%, respectively. Kairomone released by Asplanchna at 80 ind. L−1 increased the intrinsic rate of population increase by 15.8%, but that at 160 ind. L−1 decreased the intrinsic rate of population increase by 19.8%. Kairomone released by Asplanchna at 80–160 ind. L−1 increased the proportion of sexual offspring by 386.7% and 426.7%, respectively. In addition, compared to 1.0 × 106 cells · mL−1 of S. obliquus, and when B. angularis was exposed to kairomone released by Asplanchna at 160 ind. L−1, 2.0 × 106 cells · mL−1 of S. obliquus decreased the life expectancy at hatching and the average lifespan by 18.6% and 21.1%, respectively. When B. angularis was exposed to kairomone released by Asplanchna at 40 and 160 ind. L−1, 2.0 × 106 cells · mL−1 of S. obliquus decreased the intrinsic rate of population increase by 6.88% and 18.5%, respectively (Fig. 2).
Two-way ANOVA showed that both the life expectancy at hatching and the average lifespan of B. angularis were significantly affected by Asplanchna-released kairomone concentration, food level and their interaction. Both the generation time and the proportion of sexual offspring were affected by Asplanchna-released kairomone concentration. The net reproduction rate and the intrinsic rate of population increase were affected by both Asplanchna-released kairomone concentration and food level (Tab. 1).
 |
Fig. 1 Age-specific survivorship (filled square) and fecundity (unfilled triangle) of B. angularis exposed to kairomone concentrations released respectively by four densities of Asplanchna and cultured at two S. obliquus levels. Shown are the values mean + standard error based on three replicates. |
 |
Fig. 2 Life expectancy at hatching, the average lifespan, generation time, net reproduction rate, intrinsic rate of population increase and proportion of sexual offspring of B. angularis exposed to kairomone concentrations released respectively by four densities of Asplanchna and cultured at two S. obliquus levels. Shown are the values mean + standard error based on three replicates. Small and capital letters indicate means that are similar (same letter) or different (different letters) for each variable among four Asplanchna-released kairomone concentrations when fed 1.0 × 106 (unfilled bars) and 2.0 × 106 (filled bars) cells mL−1 of S. obliquus, respectively (LSD multiple comparison), and asterisk (*) indicates means that are different for each variable between two food levels (P < 0.05, t-test). |
Results of analysis of variance (two-way ANOVA) performed for each life-table demographic parameter of B. angularis exposed to four Asplanchna-released kairomone concentrations and cultured at two S. obliquus levels.
3.2 Population growth
At both algal levels, Asplanchna-released kairomone concentration affected significantly the rate of population growth (P < 0.05), but did not influenced lorica length and width, body size and egg volume of B. angularis (P > 0.05). Compared with the controls, and at 1.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 160 ind. L−1 decreased the rate of population growth by 26.5%. At 2.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 40 ind. L−1 increased the rate of population growth by 6.75%, but that at 160 ind. L−1 decreased it by 6.75% (Fig. 3).
Two-way ANOVA showed that the rate of population growth of B. angularis was significantly affected by Asplanchna-released kairomone concentration, food level and their interaction. Both lorica length and egg volume of B. angularis were affected only by the interaction between Asplanchna- released kairomone concentration and food level (Tab. 2).
 |
Fig. 3 Rate of population growth, lorica length and width, body size and egg volume of B. angularis exposed to kairomone concentrations released respectively by four densities of Asplanchna and cultured at two S. obliquus levels. Shown are the values mean + standard error based on three replicates. Small and capital letters indicate means that are similar (same letter) or different (different letters) for each variable among four Asplanchna-released kairomone concentrations when fed 1.0 × 106 (unfilled bars) and 2.0 × 106 (filled bars) cells mL−1 of S. obliquus, respectively (LSD multiple comparison). |
Results of analysis of variance (two-way ANOVA) performed for rate of population growth and morphometric parameters of B. angularis exposed to four Asplanchna-released kairomone concentrations and cultured at two S. obliquus levels.
4 Discussion
Predation is recognized as a major factor affecting the life history traits of freshwater rotifers (reviewed in Gilbert, 2013). In order to understand life history strategies of rotifers under Asplanchna predation, both life table approach and renewed batch culture method are usually simultaneously used. When rotifer females of basic morph (parental females) were used as subject, life table approach was used to study its survival, reproduction and population growth under Asplanchna predation, but most population growth experiments included multiple, overlapping generations of rotifers, which would lead to inaccurate analyses on life-history strategies, because females of basic morph were not subject to possible allocation costs related to bearing longer, induced spines during postnatal growth and throughout their lifetime, but the proportion of induced morphs in the Asplanchna treatments was initially zero and then would have gradually increased over time as these females reproduced (reviewed in Gilbert, 2013). Gilbert (2013) proposed that the interval for population growth experiments has to be short, probably similar to the lifespan of the rotifer. Considering the short juvenile period (1.25 d) and fast embryonic development (0.73 d) of B. angularis at 20 °C (Walz, 1987), the present study allowed the rotifer populations to grow for only 3 days, and then obtained the population growth rate, and morphometric characters of the parental females and their egg sizes. Three-day population growth experiment is also usually used to test the effects of environmental factors such as temperature, salinity, food level and toxicant concentration on the population growth rates of rotifers (e.g. Snell, 1986; Radix et al., 2002; Xi and Feng, 2004; Xi et al., 2007).
Survivorship and offspring production are the two key components of fitness (Case, 2000; Stelzer, 2005). The studies on females of basic morph of several rotifer species showed that both the survival and the reproductive rate of rotifers were affected not only by temperature, food level and predator- released kairomone concentration, but also by rotifer species. At 15 °C under same food levels, both the survival and the reproductive rate of B. havanaensis Rousselet decreased significantly with increasing Asplanchna-released kairomone concentration; but at higher temperature and higher food density, they were not affected by Asplanchna-released kairomone concentration (Pavón-Meza et al., 2008). The survival (average lifespan and life expectancy at hatching) of B. calyciflorus decreased in the presence of kairomone released by A. brightwellii at 800 ind. L−1, but that of Plationus patulus macracanthus Daday (Segers, 2007) did not. The reproductive rate of B. calyciflorus decreased significantly with increasing Asplanchna-released kairomone concentration, and that of P. patulus macracanthus decreased in the presence of kairomone released by A. brightwellii at 800 ind. L−1 (Sarma et al., 2011). The present study showed that at 1.0 × 106 cells · mL−1 of S. obliquus, kairomone released by Asplanchna at 40–160 ind. L−1 decreased the average lifespan and the life expectancy at hatching, and that released by Asplanchna at 80–160 ind. L−1 decreased the net reproduction rate of parental B. angularis females. The increase in food level decreased the effect magnitude of kairomone released by Asplanchna at 40 ind. L−1 on the average lifespan and the life expectancy at hatching, but increased that on the net reproduction rate.
Reproduction in one age class can be deleterious to parental survival to subsequent age classes. A greater investment in reproduction by rotifers often lowers survivorship (Sarma et al., 2002; Ogello et al., 2016). In the presence of kairomone released by salamander axolotl Ambystoma mexicanum Shaw & Nodder, B. havanaensis adopted the life history strategy of high reproduction and low survivorship; but in the presence of kairomone released by copepod Acanthocyclops robustus Sars, it adopted the opposite life history strategy of low reproduction and high survivorship (García et al., 2007). B. calyciflorus exposed to Asplanchna-conditioned medium adopted the life history strategy of low reproduction and high survivorship (Guo et al., 2011). However, in the present study, we did not find that B. angularis allocated energy between reproduction and survivorship, but increased investment in sexual reproduction was observed.
The kairomones released by fish (which prefer larger prey) cause an earlier reproduction, production of more but smaller eggs and overall body size reduction in Daphnia (Vanni, 1987; Machádek, 1991; Stibor, 1992). In the presence of kairomones released by invertebrate predators (which prefer smaller prey), individuals of some Daphnia clones delay their maturation and produce fewer but larger offspring (Spitze, 1991; Brett, 1992; Lüning, 1992; Pijanowska and Kowalczewski, 1997). In the present study, kairomone released by A. brightwelli did cause an earlier reproduction (shortened generation time) and production of fewer eggs (inferred from decreased net reproduction rate), but it did not affect body sizes of parental B. angularis females and their egg volumes. Therefore, whether energy expenditure in survival and reproduction of parental B. angularis females was saved and allocated to the hidden morphological defenses needs further investigation.
The population growth rate of rotifers generally decreased in the presence of Asplanchna kairomone (e.g. Pavón-Meza et al., 2008; Aránguiz-Acuña et al., 2010; Sarma et al., 2011). However, the population growth rate of B. havanaensis increased in the presence of kairomone released by A. mexicanum or A. robustus (García et al., 2007). In the present study, we found that B. angularis cultured at 2 × 106 cells · mL−1 of S. obliquus developed a significantly high intrinsic rate of population increase in the presence of kairomone released by Asplanchna at 80 ind. L−1 (the life table experiments) and a markedly high population growth rate in the presence of kairomone released by Asplanchna at 40 ind. L−1 (the population growth experiments), which supports the hypothesis that B. angularis develops a high population growth rate in response to kairomone released by a certain density of Asplanchna. The lower Asplanchna density resulting in the significantly high population growth rate of B. angularis might be attributed to accumulation of Asplanchna kairomone in the un-renewed medium during the three-day population growth experiments.
Gilbert (2013) thought that higher Asplanchna densities may produce higher concentrations of excretory products which may inhibit, or promote, rotifer population growth. The results available now showed that higher densities of A. brightwelli promoted indirectly not only the population growth but also the lorica thickness and hardness of B. angularis (the resent study; Yin et al., 2017). It might be difficult to conclude that all those indirect effects result from Asplanchna excretory products. The most ideal methodology for determining the effects of kairomone on population growth rates of rotifers would involve the use of both purified kairomone and chemostat cultures (Gilbert, 2013).
Pourriot (1986) thought that the rate at which planktonic rotifers multiply during the parthenogenetic phase, providing there is sufficient food, is due more to the short period of embryonic development and the early period of life than to the net reproduction rate. The kairomone released by copepod A. robustus decreased the generation time and thus increased the population growth rate of B. havanaensis (García et al., 2007). Identical results were obtained in the present study, which supported the hypothesis that kairomone released by a certain density of Asplanchna shortens the generation time and thus increases the population growth rate of B. angularis.
As a predator avoidance strategy, diapause in D. magna can be induced by fish kairomones to avoid the predator in time instead of facing the enemy through defenses (Ślusarczyk, 1995, 1999, 2001; Pijanowska and Stolpe, 1996). In the presence of kairomone released by A. brightwellii at 100 ind. L−1, B. calyciflorus saves energy expenditure in sexual reproduction and allocates it to the production of more parthenogenetic offspring to offset predation loss, but B. angularis preserves the energy expenditure of sexual reproduction and maintains resting-egg production (Yin et al., 2017). The present study showed that B. angularis exposed to the kairomone released by A. brightwellii at 80 and 160 ind. L−1 invested more energy in sexual reproduction, which supported the hypothesis that higher concentrations of Asplanchna kairomone induce higher levels of sexual reproduction, and verified the idea that under heavy predation pressure, with a low chance of survival for parthenogenetic females, resting egg formation may result in a higher fitness than immediate reproduction (Pijanowska and Stolpe, 1996).
5 Conclusion
Asplanchna-released kairomone decreased significantly average lifespan, life expectancy at hatching, generation time and net reproduction rate, but increased the proportion of sexual offspring of parental B. angularis females. The threshold Asplanchna density required for significant effects varied with food level. Kairomone released by Asplanchna at 80 ind. L−1 increased significantly the intrinsic rate of population increase of B. angularis, which would offset the mortality of exposed females from predation. The accumulation of kairomone in aquatic environments enhanced the indirect effect of Asplanchna on the population growth of B. angularis. Rapid population growth of B. angularis induced by Asplanchna kairomone might facilitate the coexistence of preys with predators, and higher proportion of sexual offspring and then resting egg production might help the preys avoid the predator in time instead of facing the enemy through defenses.
Acknowledgements
We thank the Shenzhen Nobel Science and Technology Service Co., Ltd. for language editing service. This work was funded by the Natural Science Foundation of China (31470015, 31170395) and the Foundation of Provincial Key Laboratory of Biotic Environment and Ecological Safety in Anhui Province.
References
- Aránguiz-Acuña A, Ramos-Jiliberto R, Sarma N, Sarma SSS, Bustamante RO, Toledo V. 2010. Benefits, costs and reactivity of inducible defences: an experimental test with rotifers. Freshwat Biol 55: 2114–2122. [CrossRef] [Google Scholar]
- Brett MT. 1992. Chaoborus and fish mediated influences on Daphnia longispina population structure, dynamics and life history strategies. Oecologia 89: 69–77. [CrossRef] [PubMed] [Google Scholar]
- Case TJ. 2000. An Illustrated Guide to Theoretical Ecology, Oxford: Oxford University Press. [Google Scholar]
- De Meester L, Dawidowicz P, van Gool E, Loose CJ. 1999. Ecology and evolution of predator-induced behavior of zooplankton: depth selection behavior and diel vertical migration. In: Tollrian R, Harvell CD, eds. The Ecology and Evolution of Inducible Defenses. Princeton, NJ: Princeton University Press, pp. 160–176. [Google Scholar]
- Dumont HJ, Sarma SSS. 1995. Demography and population growth of Asplanchna girodi (Rotifera) as a function of prey (Anuraeopsis fissa) density. Hydrobiologia 306: 97–107. [CrossRef] [Google Scholar]
- García CE, Chaparro-Herrera DJ, Nandini S, Sarma SSS. 2007. Life history strategies of Brachionus havanaensis subject to kairomones of vertebrate and invertebrate predators. Chem Ecol 23: 303–313. [CrossRef] [Google Scholar]
- Gilbert JJ. 1963. Mictic female production in rotifer Brachionus calyciflorus. J Exp Zool 153: 113–124. [CrossRef] [Google Scholar]
- Gilbert JJ. 1967. Asplanchna and posterolateral spine induction in Brachionus calyciflorus. Arch Hydrobiol 64: 1–62. [Google Scholar]
- Gilbert JJ. 1999. Kairomone-induced morphological defenses in rotifers. In: Tollrian R, Harvell CD, eds. The Ecology and Evolution of Inducible Defenses. Princeton, New Jersey: Princeton University Press, pp. 127–141. [Google Scholar]
- Gilbert JJ. 2013. The cost of predator-induced morphological defense in rotifers: experimental studies and synthesis. J Plankt Res 35: 461–472. [CrossRef] [Google Scholar]
- Gilbert JJ. 2014. Morphological and behavioral responses of a rotifer to the predator Asplanchna. J Plankt Res 36: 1576–1584. [Google Scholar]
- Gilbert JJ. 2017. Non-genetic polymorphisms in rotifers: environmental and endogenous controls, development, and features for predictable or unpredictable environments. Biol Rev 92: 964–992. [CrossRef] [Google Scholar]
- Guo R, Snell TW, Yang J. 2011. Ecological strategy of rotifer (Brachionus calyciflorus) exposed to predator- and competitor-conditioned media. Hydrobiologia 658: 163–171. [CrossRef] [Google Scholar]
- Halbach U. 1970. Die Ursachen der Temporalvariation von Brachionus calyciflorus Pallas (Rotatoria). Oecologia 4: 262–318. [CrossRef] [PubMed] [Google Scholar]
- Hairston NG. 1987. Diapause as a predator avoidance adaptation. In: Kerfoot WC, Sih A, eds. Predation: Direct and Indirect Impacts on Aquatic Communities. Hanover, U.S.A.: University Press of New England, 281–290. [Google Scholar]
- Lass S, Spaak P. 2003. Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491: 221–239. [CrossRef] [Google Scholar]
- Larsson P, Dodson S. 1993. Chemical communication in planktonic animals. Arch. Hydrobiol 129: 129–155. [Google Scholar]
- Li S-H, Zhu H, Xia Y-Z, Yu M-J, Liu K-S, Ye Z, Chen Y-Y. 1959. The mass culture of unicellular green algae. Acta Hydrobiol Sin 4: 462–472. [Google Scholar]
- Lüning J. 1992. Phenotypic plasticity of Daphnia pulex in the presence of invertebrate predators: morphological and life history responses. Oecologia 92: 383–390. [CrossRef] [PubMed] [Google Scholar]
- Machádek J. 1991. Indirect effect of planktivorous fish on the growth and reproduction of Daphnia galeata. Hydrobiologia 225: 193–197. [CrossRef] [Google Scholar]
- Ogello EO, Kim H-J., Suga K, Hagiwara A. 2016. Life table demography and population growth of the rotifer Brachionus angularis in Kenya: influence of temperature and food density. Afr J Aquat Sci 41: 329–336. [CrossRef] [Google Scholar]
- Pavón-Meza EL, Sarma SSS, Nandini S. 2008. Combined effects of temperature, food availability and predator's (Asplanchna girodi) allelochemicals on the demography and population growth of Brachionus havanaensis (Rotifera). Allelopathy J 21: 95–106. [Google Scholar]
- Peña-Aguado F, Morales-Ventura J, Nandini S, Sarma SSS. 2008. Influence of vertebrate and invertebrate infochemicals on the population growth and epizoic tendency of Brachionus rubens (Ehrenberg) (Rotifera: Brachionidae). Allelopathy J 22: 123–130. [Google Scholar]
- Peng B, Cao H-Y, Pan L, Xi Y-L. 2016. Clonal diversity of population growth parameter of Brachionus angularis from Lake Jinghu. J. Anhui Normal Univ. (Nat Sci) 39: 391–376. [Google Scholar]
- Pianka ER. 1988. Evolutionary Ecology (3rd edn). New York: Harper & Row. [Google Scholar]
- Pijanowska J, Stolpe G. 1996. Summer diapause in Daphnia as a reaction to the presence of fish. J Plank Res 18: 1407–1412. [CrossRef] [Google Scholar]
- Pijanowska J, Kowalczewski A. 1997. Cues from injured Daphnia and from cyclopoids feeding on Daphnia can modify life histories of conspecifics. Hydrobiologia 350: 99–103. [CrossRef] [Google Scholar]
- Poole RW. 1974. An Introduction to Quantitative Ecology. New York: McGraw-Hill. [Google Scholar]
- Pourriot R. 1986. Les rotifers − biologie. Aquaculture 5: 201–221. [Google Scholar]
- Radix P, Severin G, Schramm KW, Kettrup A. 2002. Reproduction disturbances of Brachionus calyciflorus (rotifer) for the screening of environmental endocrine disruptors. Chemosphere 47: 1097–1101. [CrossRef] [PubMed] [Google Scholar]
- Sarma SSS, Nandini S, Gulati RD. 2002. Cost of reproduction in selected species of zooplankton (rotifers and cladocerans). Hydrobiologia 481: 89–99. [CrossRef] [Google Scholar]
- Sarma SSS, Resendiz RAL, Nandini S. 2011. Morphometric and demographic responses of brachionid prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwelli (Rotifera: Asplanchnidae). Hydrobiologia 662: 179–187. [CrossRef] [Google Scholar]
- Segers H. 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564: 1–104. [Google Scholar]
- Ślusarczyk M. 1995. Predator-induced diapause in Daphnia. Ecology 76: 1008–1013. [CrossRef] [Google Scholar]
- Ślusarczyk M. 1999. Predator-induced diapause in Daphnia magna may require two chemical cues. Oecologia 119: 159–165. [CrossRef] [PubMed] [Google Scholar]
- Ślusarczyk M. 2001. Food threshold for diapause in Daphnia under the threat of fish predation. Ecology 82: 1089–1096. [CrossRef] [Google Scholar]
- Snell TW. 1986. Effect of temperature, salinity and food level on sexual and asexual reproduction in Brachionus plicatilis (Rotifera). Mar. Biol. 92: 157–162. [CrossRef] [Google Scholar]
- Soto CS, Sarma SSS. 2009. Morphometric changes in Lecane stokesii (Pell, 1890) (Rotifera: Lecanidae) induced by allelochemicals from the predator Asplanchnopus multiceps (Schrank, 1793). Allelopathy J. 24: 215–222. [Google Scholar]
- Spitze K. 1991. Chaoborus predation and life-history evolution in Daphnia pulex: temporal pattern of population diversity, fitness, and mean life history. Evolution 45: 82–92. [CrossRef] [PubMed] [Google Scholar]
- Stelzer C. 2005. Evolution of rotifer life histories. Hydrobiologia 546: 335–346. [CrossRef] [Google Scholar]
- Stibor H. 1992. Predator induced life-history shifts in a freshwater cladoceran. Oecologia (Berlin) 92: 162–165. [Google Scholar]
- Tollrian R, Dodson SI. 1999. Inducible defenses in Cladocera: constraints, costs, and multipredator environments. In: Tollrian R, Harvell CD, eds. The Ecology and Evolution of Inducible Defenses. New York Princeton, NJ: Princeton University Press, 177–202. [Google Scholar]
- Vanni MJ. 1987. Indirect effect of predators on age-structured prey populations: planktivorous fish and zooplankton. In: Kerfoot WC, Sih A, eds. Predation: Direct and indirect impacts on aquatic communities. Hanover, New Hampshire: New England Press, 149–160. [Google Scholar]
- Wallace RL, Snell TW, Ricci C. 2006. Rotifera. Vol 1: Biology, ecology and systematics. In: Segers H, Dumont HJF, eds. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 23, Kenobi Productions. The Hague: Ghent/Backhuys Academic Publishing. [Google Scholar]
- Walz N. 1987. Comparative population dynamics of the rotifers Brachionus angularis and Keratella cochlearis. Hydrobiologia 147: 209–211. [CrossRef] [Google Scholar]
- Williams DD. 1997. Temporary ponds and their invertebrate community. Aquat Conserv Mar Freshwater Ecosyst 7: 105–117. [Google Scholar]
- Xi Y-L, Feng L-K. 2004. Effects of thiophanate-methyl and glyphosate on asexual and sexual reproduction in the rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 73: 644–651. [PubMed] [Google Scholar]
- Xi Y-L, Chu Z-X, Xu X-P. 2007. Effect of four organochlorine pesticides on the reproduction of freshwater rotifer Brachionus calyciflorus Pallas. Environ Toxicol Chem 26: 1695–1699. [CrossRef] [PubMed] [Google Scholar]
- Xie P, Xi Y-L, Wen X-L, Zhou J, Li Y, Niu X-X, Wang A-M, Wang J-X. 2015. Responses of the spatio-temporal dynamics of rotifer community structure to the concentrations of N and P, and the effect of top-down in two lakes. Acta Ecol Sin 35: 4763–4776. [Google Scholar]
- Yin XW, Zhou YC, Li XC, Li WX. 2015. Reduced investment in sex as a cost of inducible defence in Brachionus calyciflorus (Rotifera). Freshwat Biol 60: 89–100. [Google Scholar]
- Yin XW, Jin W, Zhou YC, Wang PP, Zhao W. 2017. Hidden defensive morphology in rotifers: benefits, costs, and fitness consequences. Sci Rep, 7, 4488. [CrossRef] [PubMed] [Google Scholar]
- Zhang Y, Zhou A, Xi Y-L, Sun Q, Ning L-F, Xie P, Wen X-L, Xiang X-L. 2017. Temporal patterns and processes of genetic differentiation of the Brachionus calyciflorus (Rotifera) complex in a subtropical shallow lake . Hydrobiologia DOI:10.1007/s10750-017-3407-9. [PubMed] [Google Scholar]
- Zhang ZS, Huang XF. 1991. Method for Study on Freshwater Plankton Science Press, Beijing. [Google Scholar]
Cite this article as: Pan L, Xi Y-L, Gu J, Jiang S, Zhu H, Zhang B-X. 2018. Asplanchna-kairomone induces life history shifts in Brachionus angularis (Rotifera). Ann. Limnol. - Int. J. Lim. 54: 13
All Tables
Results of analysis of variance (two-way ANOVA) performed for each life-table demographic parameter of B. angularis exposed to four Asplanchna-released kairomone concentrations and cultured at two S. obliquus levels.
Results of analysis of variance (two-way ANOVA) performed for rate of population growth and morphometric parameters of B. angularis exposed to four Asplanchna-released kairomone concentrations and cultured at two S. obliquus levels.
All Figures
 |
Fig. 1 Age-specific survivorship (filled square) and fecundity (unfilled triangle) of B. angularis exposed to kairomone concentrations released respectively by four densities of Asplanchna and cultured at two S. obliquus levels. Shown are the values mean + standard error based on three replicates. |
| In the text | |
 |
Fig. 2 Life expectancy at hatching, the average lifespan, generation time, net reproduction rate, intrinsic rate of population increase and proportion of sexual offspring of B. angularis exposed to kairomone concentrations released respectively by four densities of Asplanchna and cultured at two S. obliquus levels. Shown are the values mean + standard error based on three replicates. Small and capital letters indicate means that are similar (same letter) or different (different letters) for each variable among four Asplanchna-released kairomone concentrations when fed 1.0 × 106 (unfilled bars) and 2.0 × 106 (filled bars) cells mL−1 of S. obliquus, respectively (LSD multiple comparison), and asterisk (*) indicates means that are different for each variable between two food levels (P < 0.05, t-test). |
| In the text | |
 |
Fig. 3 Rate of population growth, lorica length and width, body size and egg volume of B. angularis exposed to kairomone concentrations released respectively by four densities of Asplanchna and cultured at two S. obliquus levels. Shown are the values mean + standard error based on three replicates. Small and capital letters indicate means that are similar (same letter) or different (different letters) for each variable among four Asplanchna-released kairomone concentrations when fed 1.0 × 106 (unfilled bars) and 2.0 × 106 (filled bars) cells mL−1 of S. obliquus, respectively (LSD multiple comparison). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


