| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 54, 2018
|
|
|---|---|---|
| Article Number | 16 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/limn/2018006 | |
| Published online | 17 April 2018 | |
Research Article
Effects of food concentration on the life table demography and morphology of three Keratella quadrata morphotypes
Provincial Key Laboratory for Conservation and Utilization of Important Biological Resource in Anhui, College of Life Sciences, Anhui Normal University,
Wuhu,
Anhui,
241000, PR China
* Corresponding author: ylxi1965@126.com
Received:
10
October
2017
Accepted:
25
January
2018
Keratella quadrata with two (2PS), one (1PS) and none posteolateral (0PS) spines were cultured under four food levels (0.75 × 106, 1.5 × 106, 3.0 × 106 and 6.0 × 106 cells·mL−1 of Scenedesmus obliquus) to test the differences in the life table demography and the morphological characteristics among these three morphotypes. The results showed that each K. quadrata morphotype could produce 2PS, 1PS and 0PS offsprings. The frequencies of 0PS were extremely low (<5%) and could be considered as a small probability event, suggesting that the 0PS morphotype might be an abnormal status. The following life table demographic tests suggested that 0PS morphotype had a relatively lower intrinsic rate of population growth at high food concentrations and a relatively lower average lifespan, in comparison to 2PS rotifers. These results further supported that the 0PS K. quadrata might be an abnormal development. Along with the elevating food concentration, 1PS morphotype reproduced more 2PS offsprings, suggesting that high energy input might be helpful to grow more posterolateral spines. However, in response to the increasing food concentration, 0PS rotifers produced more 1PS offsprings. The underlying mechanisms required further investigations. The posterolateral spine length of offsprings of 0PS K. quadrata was significantly longer than those of 2PS and 1PS rotifer parents at the four food concentrations, which probably help the offsprings of 0PS rotifer parents to survive in natural environments, since long and more posterolateral spines offer rotifers high ability to compete with other rotifers and cladocerans for food or to resist predators.
Key words: food concentration / life history traits / morphological traits / rotifer
© EDP Sciences, 2018
1 Introduction
Polymorphism is a widely phenomenon in planktonic rotifers, especially in Brachionus, Keratella and Asplanchna. The morphological alteration in rotifers is considered as developmental responses to various types of environmental factors (Eloranta, 1982; Garza-Mouriño et al., 2005; Ge et al., 2012; Green, 2007; Hillbricht-Ilkowska, 1983; Pavön-Meza et al., 2005; Xi et al., 2002). Rotifers with different morphological characters show different fitness and adaptation to a certain condition.
As a commonly known morphological character, the posterolateral spine of Brachionus and Keratella varies remarkably. Rotifers may have two, one or none posterolateral spines (2PS, 1PS and 0PS, respectively). The posterior spine of rotifers is generally considered as a defense strategy to predators. Emergence of predators or predator-mediated media induces more and longer posterior spines. For example, Asplanchna could induce 0PS, 1PS and 2PS morphotypes of B. calyciflorus to reproduce two long-spined morphotype (Yin and Niu, 2007). Moreover, without the effects of Asplanchna, 0PS, 1PS and 2PS of B. calyciflorus could coexist under laboratory conditions, and each morphotype could produce all the three morphotypes of females (Yin and Niu, 2007). These results suggested that the different morphotypes could transform mutually. The underlying ecological mechanisms remain largely unknown.
Previously, researchers hypothesized that different morphotypes of rotifers might have different fitness. For example, Stemberger (1988) and Sarma et al., (2011) revealed that K. testudo and B. calyciflorus with different posterolateral spine length differed in their population growth rates. Similarly, Athibai and Sanoamuang (2008) found that the duration of post-reproductive and juvenile period of B. caudatus f. apsteini offsprings varied with increasing posterolateral spine length. In contrast, Gilbert (2012) found that population growth rates of B. calyciflorus and K. tropic did not change with increasing posterolateral spine length. These inconsistent results might be attributed to environmental conditions and the tested species. More tests on different rotifer species under gradients (such as gradient food level) would be helpful to compare the environmental preference of different morphotypes and further test this hypothesis.
Field investigations revealed that, besides of predators, food also influenced the presence and length of posterolateral spine, the lorica length and width of Keratella and Brachionus (Hillbricht-Ilkowska, 1983; Gilbert, 1999; Pejler, 1980). In the present study, the effects of food density on the life table demography and morphological characters of offsprings were compared among three K. quadrata morphotypes. We aimed to (1) test the effects of food density on the transformation of different morphotypes, and (2) explore whether different morphotypes had preferences to food concentration. The results would contribute to better understand the ecological mechanisms underlying the transformation of different morphotypes of rotifers.
2 Materials and methods
2.1 Rotifer collection and culture
K. quadrata were collected during winter 2009 from Lake Tingtang located in a park in the center of Wuhu City, Anhui Province, P. R. China (119°21′E. 31°20′N). Individuals with amictic eggs were randomly selected, and cultured clonally in a light incubator (PGX-350B, Ningbo Saifu Experimental Equipment Company Limited, China) at 10 ± 1 °C with a light: dark photoperiod of 16 h:8 h and the light intensity was approximately 130 lx (similar to field conditions at sampling date). Rotifer medium contained 100 mg/L KNO3, 40 mg/L K2HPO4, 62 mg/L MgSO4·7H2O and 144 mg/L Ca(NO3)2·4H2O and 2mM NaH2PO4–Na2HPO4 buffer (pH 7.3) (Gilbert, 1963). Rotifers were fed 1.0 × 106 cells·mL−1 Scenedesmus obliquus daily. The algae were grown semi-continuously in the HB-4 medium (Li et al., 1959). Log-phase algae were harvested, concentrated by centrifugation at 3,000 rpm for 10 min and resuspended in rotifer culture medium. The algal density was measured using a haemocytometer (XB-K-25, Shanghai Huake Experimental Equipment Company Limited, China). After 20 successive parthenogenetic generations, 12 clones of K. quadrata during the exponential growth were randomly selected. They were precultured under four food concentrations (0.75 × 106, 1.5 × 106, 3.0 × 106 and 6.0 × 106 cells·mL−1) of S. obliquus in 10 mL test tubes for at least seven days. Rotifers were transferred to new test tubes using a glass dropper daily. Around one third to one half of culture medium was replaced using a glass dropper covered with 25 µm pore-sized net at the open end.
Four clones of each morphotype (2PS, 1PS and 0PS) were used in this test. From each clone, >200 animals with amictic eggs were collected and transferred to a dish containing medium. They were divided into three groups based on the presence of posterolateral spines. After four hours, 12 newly hatched neonates (<4 h old) were collected and then cultured in a small plastic container containing rotifer medium with the set food concentrations. Every 12 hours, the neonates were counted and then removed, and the survivor of the original animals in each container was checked under a Motic stereomicroscope device. Every day the original individuals were transferred to a new container with fresh media and algae. The experiment was continued until all original individuals died.
2.2 Measurement of morphological characters
Another 50 neonates (<4 hr old) were transferred into a 50 mL transparent jar containing 30 mL of rotifer medium and were cultured under the same conditions as stated above. The animals were harvested to measure morphometric characteristics when they produced the first amictic egg. Lorica length without considering anterior and posterior spine, lorica width and posterior spine length (average length of left and right posterolateral spine for 2PS K. quadrata) of each rotifer were measured using a Motic 3.0 Images Device at the magnification of 400 × (Diéguez et al., 1998).
2.3 Data analyses
According to Krebs (1985), Pianka (1988) and Charlesworth (1994) the main life history variables were calculated, including age-specific survivorship (lx) and fecundity (mx), net reproductive rate (R0), intrinsic rate of population growth (rm), average lifespan, generation time (G0: cohort generation time; G1: time necessary for a population to increase by R0; Gh: parental age at the production of a new generation), population doubling time (PDT), and intrinsic rate of population growth (rm) and average lifespan (AL).
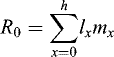 Intrinsic rate of population growth (r) was first approximated using:
Intrinsic rate of population growth (r) was first approximated using:
 For final calculation, we solved the equation:
For final calculation, we solved the equation: 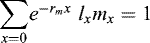
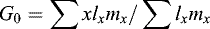
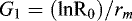
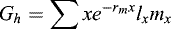
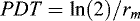 where:
where:
x = time [h]
lx = age-specific survivorship
mx = age-specific fecundity
All data were tested for normality using the one-sample Kolmogórov-Smirnov procedure. Homogeneity of variances was checked using the Levene's test. One-way ANOVA was conducted to identify the significant effects of food concentration and parental morphotype on life table demography parameters and morphological characteristics. Two-way ANOVA was conducted to reveal the significant effects of food concentration, parental morphotype and the interaction of food concentration × parental morphotype on the morphological characteristics. Multiple comparisons (SNK-q test) were conducted to identify groups that were significantly different.
3 Results
The fecundity peak of the three parental morphotypes differed with food concentration. At 0.75 × 106 and 1.5 × 106 cells·mL−1, the fecundity curves of the three morphotypes were similar. At 3.0 × 106 and 6.0 × 106 cells·mL−1, the fecundity peaks of 2PS and 1PS rotifers were relatively higher than that of 0PS morphotype (Fig. S1).
At 0.75 × 106 cells·mL−1, the age-specific survivorship of 2PS rotifers declined more slowly than those of other two morphotypes. In comparison, the decline of survivorship of 1PS morphotype was relatively slower at 1.5 × 106 cells·mL−1. The survivorship curves were similar among the three morphotypes at both 3.0 × 106 and 6.0 × 106 cells·mL−1 (Fig. S1).
The net reproductive rate, the intrinsic rate of population growth and the generation time were all not affected significantly by parental morphotype and food concentration (P < 0.05). In comparison, the average lifespan differed among the three morphotypes and among the four food concentrations. At 0.75 × 106 cells·mL−1, the average lifespan of 2PS rotifers was longer than those of 1PS and 0PS rotifers. At 1.5 × 106 and 3.0 × 106 cells·mL−1, the 0PS rotifers revealed the lowest average lifespan (Tab. 1).
The 2PS and 0PS rotifers showed a relatively longer lifespan at 0.75 × 106 cells·mL−1, compared with other food concentrations. No significant differences were observed in the lifespan of 1PS rotifers among the four tested food concentrations (Tab. 1).
Two-way ANOVA analyses showed that only morphotype significantly affected the population doubling time and the average lifespan but did not affect other life-table parameters. Food concentration and the interaction between morphotype and food concentration did not significantly influence all the tested life-table parameters (Table S1).
The tested morphological characters, including lorica length, lorica width and posterolateral spine length significantly responded to food concentration and parental morphotype (Tab. 2). For 2PS rotifer parent, the lorica length of offsprings was longer at 3.0 × 106 cells·mL−1 than that at 0.75 × 106 cells·mL−1, but the lorica width and posterolateral spine length did not change with food concentration. For 1PS and 0PS rotifer parents, the lorica width and posterolateral spine length of offsprings were relatively shorter at low food concentration than those at high food concentration, but lorica length of offsprings was not affected by food concentration (Tab. 2).
At 0.75 × 106 cells·mL−1, offsprings produced by 2PS rotifer parent revealed the longest lorica width among different morphotypes. At 6.0 × 106 cells·mL−1, offsprings produced by 0PS rotifer parent showed the longest lorica length, and offsprings produced by 1PS rotifer parent had the shortest lorica width. At all tested food concentrations, 0PS rotifer parent produced offsprings with the longest posterior spine length than those of 2PS and 1PS rotifer parents (Tab. 2).
The proportion of offspring morphotypes were calculated. At all food concentrations, rotifer parent produced various morphotypes. Among them, most offsprings were 2PS. Along with the increasing food concentration, 1PS rotifer parent tended to produce more 2PS offsprings, with the proportion increasing from 73.9 ± 6.3% to 95.7 ± 3.2%. For 0PS rotifer parent, the proportion of 1PS offspring increased from 3.8 ± 2.1% to 20.7 ± 4.6% (Tab. 2).
Two-way analysis of variance (Table S2) showed that food concentration affected significantly lorica length, lorica width and posterolateral spine length of offsprings. Rotifer parental morphotype affected significantly all the morphological characters and proportion of 2PS and 1PS offsprings. The interaction between food concentration and rotifer parental morphotype only affected the posterolateral spine length of offsprings.
Life-table demography of offsprings produced by three K. quadrata morphotypes at four food concentrations (×106 cells·mL−1) (mean ± SE). 2PS: rotifer with two posterolateral spines; 1PS: rotifer with one posterolateral spine; 0PS: rotifer with none posterolateral spine. The different lowercase and capital letters represent significant differences among different concentrations and among three morphs, respectively. R0: net reproductive rate; rm: intrinsic rate of population growth; G0/G1/Gh: alternative measures of the generation time; PDH: population doubling time, AL: average lifespan.
Morphological characters of offsprings produced by three K. quadrata morphtypes at four food concentrations (×106 cells·mL−1) (mean ± SE). 2PS: rotifer with two posterolateral spine; 1PS: rotifer with one posterolateral spine; 0PS: rotifer with none posterolateral spine. The different lowercase and capital letters represent significant differences among different concentrations and among three morphs, respectively.
4 Discussion
The frequency and transformation of offspring morphotype in rotifera is an old topic. Hatched from B. calyciflorus resting eggs, most 1st generation offsprings were 0PS morphotype, and in the subsequent generations, the ratio of 0PS morphotype decreased with the increase of 2PS morphotype (Yin and Niu, 2008). 0PS morphotype might be more energy-economic, thus the 1st generation offspring could prevail rapidly or survive longer. In the following generations, with the increasing population density, morphotype with posterolateral spines was more prevalent in water to obtain higher fitness in the presence of interfering competitors or predators (Yin and Niu, 2008). In comparison, K. quadrata behaved in a completely different way. The 2PS morphotype dominated in the 1st generation rotifers of K. quadrata and the spine length of their offsprings decreased gradually along with the advanced generations, which was attributed to the endogenous factors (Pejler, 1980). In the present study, despite of the morphotype of rotifer parent and the food concentration, the frequency of 0PS offsprings was very low (<5%), which could be considered as a small probability event, suggesting that the 0PS morphotype of K. quadrata might be an abnormal status.
Every living system has a finite amount of resources that need to be allocated to complete life functions, such as self-maintenance and reproduction (Roff, 2002; Stearns, 1992). In rotifers, phenotypic variations might affect the costs of self-maintenance and reproduction in rotifer. The formation and growth of posterior lateral spines were suggested as an energy consumptive defense against predation (Stemberger, 1988). This hypothesis could be supported by the evidence that the population growth rate of 0PS K. testudo was higher than that of 1PS and 2PS morphotypes at high food concentration (Stemberger, 1988). However, Stemberger (1990) revealed that long-spined B. calyciflorus had a significantly higher population growth rate than short-spined morphotype over a wide range of food concentrations. Thus, whether the presence of posterolateral spines affected the growth and reproduction of rotifers remained controversial. In the present study, at low and medium food concentrations, the intrinsic rate of population growth was similar between 0PS and 2PS rotifers. However, the average lifespan of 0PS rotifers were significantly lower than 2PS rotifers, suggesting that the 0PS morphotype might own less fitness than 2PS morphotype at low food concentrations. Taken together, the present data also suggested that the 0PS K. quadrata might be an abnormal status and have less fitness than 1PS and 2PS morphotypes.
Rotifer with single posterolateral spine is a special morphotype. To the best of our knowledge, there is no explanation on the ecological benefits of this morphotype. In the present study, along with the increasing food concentration, 1PS rotifer parent produced more 2PS and less 1PS offspring, which might be due to the more energy input from more algal food. However, in the offsprings of 0PS rotifer parent, the ratio of 2PS offspring decreased and the ratio of 1PS rotifers increased, along with the elevating food level. At the current stage, this phenomenon was difficult to interpret. One possible mechanism was that the 1PS morphotype might be a tradeoff between energy cost and the competitive ability for food or resistance to predation. Rotifers with single posterior spine required less energy investment, but could still be resistant to competitors or predators. Obviously, the data in the present study are too limited to verify this hypothesis. More investigations are required in the future to dig out the underlying reasons.
Compared with the short-spined or 0PS morphotype of Keratella and the ‘aptera’ morphotype of Polyarthra 1st generation parents, the development of long posterolateral spines and the paddles during parthenogenetic generations might increase the fitness of individuals, since long posterolateral spines could increase the buoyancy of Keratella to help them stay in the phototrophic zone with abundant food resources (Green, 2005), and also could deter the predation by predators (Stemberger and Gilbert, 1984). In the present study, the posterolateral spine of offsprings produced by 0PS rotifer parent was longer than those of 2PS and 1PS rotifers at four food concentrations, suggesting that the abnormal 0PS K. quadrata might produce females with long posterolateral spines, which might have higher fitness. Meanwhile, long posterolateral spines should need more energy investment, which might in turn decrease the intrinsic rate of population growth and average lifespan of 0PS morphotype. Moreover, in the present study, offsprings produced by 2PS rotifer parent revealed the longest lorica width among different morphotypes at 0.75 × 106 cells·mL−1. At low algal concentration, the density of prey rotifer decreased and the individual predation risk increased due to the dilution effect (“safety in numbers”), compared with the situation at high food density (Tollrian et al., 2015). 2PS was considered as a defensive morphotype. The wider lorica would be helpful to resist the capture by predators. Generally, rotifers tend to be smaller at high food density to pursue rapid reproduction. At 6.0 × 106 cells·mL−1, offsprings produced by 0PS rotifer parent showed the longest lorica length in the present study. Rotifers with longer lorica might be less competitive than 1PS and 2PS rotifers for space and resources. The underlying reason still required further investigation.
5 Conclusions
2PS, 1PS and 0PS K. quadrata did not show any preference to food concentration. The 0PS morphotype might be an abnormal status and have less fitness than 2PS morphotype. Despite the morphotype of rotifer parent, the major morphotype of offsprings was 2PS. Food level might affect the ratio of 2PS and 1PS offsprings reproduced by 0PS and 1PS rotifer parents. The underlying mechanisms required further investigations.
Author contributions
Conceived and designed the experiments: YLX. Performed the experiments: YLG JHY JM DDX. Analyzed the data: YLX YLG. Contributed reagents/materials/analysis tools: YLX YLG JHY. Wrote the paper: YLG YLX RZ.
Supplementary Material
Fig. S1 Access here
Acknowledgements
We thank the Shenzhen Nobel Science and Technology Service Co., Ltd for the comments and corrections of English on the present manuscript. This work was supported by the National Natural Science Foundation of China (#31400352, #30870369), the Provincial Natural Science Foundation of Anhui Province of China (#1408085MC66), the Key Foundation for Excellent Youth in Higher Education of Anhui Province of China (#2013SQRL013ZD), the Foundation of the Provincial Key Laboratory of Biotic Environment and Ecological Safety in Anhui, the Foundation of the Provincial Key Laboratory of Conservation and Utilization for Important Biological Resources in Anhui. All experiments reported here comply with current laws in China.
References
- Athibai S, Sanoamuang LO. 2008. Effect of temperature on fecundity, life span and morphology of long- and short-spined clones of Brachionus caudatus f. apsteini (Rotifera). Int Rev Hydrobiol 93: 690–699. [CrossRef] [Google Scholar]
- Charlesworth B. 1994. Evolutioninage-structuredpopulations. Cambridge: Cambridge University Press. [Google Scholar]
- Diéguez M, Modenutti B, Queimaliños C. 1998. Influence of abiotic and biotic factors on morphological variation of Keratella cochlearis (Gosse) in a small Andean. Hydrobiologia 387/388: 289–294. [CrossRef] [Google Scholar]
- Eloranta P. 1982. Notes on the morphological variation of the rotifer species Keratella cochlearis (Gosse) s.l. in one eutrophic pond. J Plankton Res 4: 299–312. [CrossRef] [Google Scholar]
- Garza-Mouriño G, Silva-Briano M, Nandini S, Sarma SSS, Castellanos-Páez ME. 2005. Morphological and morphometrical variations of selected rotifer species in response to predation: a seasonal study of selected Brachionid species from Lake Xochimilco (Mexico). Hydrobiologia 1: 169–179. [Google Scholar]
- Ge YL, Xi YL, Ma J, Xu DD. 2012. Spatio-temporal variation of morphometric characteristics of Brachionus forficula in relation to ecological factors. Acta Ecol Sin 36: 5034–5042. [CrossRef] [Google Scholar]
- Gilbert JJ. 1963. Mictic female production in rotifer Brachionus calyciflorus. J Exp Zool 153: 113–124. [CrossRef] [Google Scholar]
- Gilbert JJ. 1999. Kairomone-induced morphological defenses in rotifers. In: Tollrian R, Harvell CD, eds. The ecology and evolution of inducible defenses. Princeton: Princeton University Press, pp. 127–141. [Google Scholar]
- Gilbert JJ. 2012. Predator-induced defense in rotifers: developmental lags for morph transformations, and effect on population growth. Aquat Ecol 46: 475–486. [CrossRef] [Google Scholar]
- Green JJ. 2005. Morphological variation of Keratella cochlearis (Gosse) in a backwater of the River Thames. Hydrobiologia 546: 189–196. [CrossRef] [Google Scholar]
- Green JJ. 2007. Morphological variation of Keratella cochlearis (Gosse) in Myanmar (Burma) in relation to zooplankton community structure. Hydrobiologia 593: 5–12. [CrossRef] [Google Scholar]
- Hillbricht-Ilkowska A. 1983. Morphological variation of Keratella cochlearis (Gosse) in Lake Biwa, Japan. Hydrobiologia 104: 297–305. [CrossRef] [Google Scholar]
- Krebs CJ. 1985. Ecology: the experimental analysis of distribution and abundance. New York: Harper & Row Press, 800 p. [Google Scholar]
- Li SH, Zhu H, Xia YZ, et al. 1959. The mass culture of nicellular green algae. Acta Hydrobiol Sin 4: 462–472. [Google Scholar]
- Pavön-Meza EL, Sarma SSS, Nandini S. 2005. Combined effects of algal (Chlorella vulgaris) food level and temperature on the demography of Brachionus havanaensis (Rotifera): a life table study. Hydrobiologia 546: 353–360. [CrossRef] [Google Scholar]
- Pejler B. 1980. Variation in the genus Keratella. Hydrobiologia 73: 207–213. [CrossRef] [Google Scholar]
- Pianka ER. 1988. Evolutionary Ecology, 3rd ed. New York: Harper & Row Press. [Google Scholar]
- Roff DA. 2002. Life history evolution. Sunderland Massachusetts: Sinauer Associates Inc. Press. [Google Scholar]
- Sarma SSS, Resendiz RAL, Nandini S. 2011. Morphometric and demographic responses of Brachionid prey (Brachionus calyciflorus Pallas and Plationus macracanthus Daday) in the presence of different densities of the predator Asplanchna brightwelli (Rotifera: Asplanchnidae). Hydrobiologia 662: 179–187. [CrossRef] [Google Scholar]
- Stearns SC. 1992. The Evolution of life histories. Oxford: Oxford University Press. [Google Scholar]
- Stemberger RS. 1988. Reproduction costs and hydrodynamic benefits of chemically induced defenses in Keratella tesutdo. Limnol Oceanogr 33: 593–606. [CrossRef] [Google Scholar]
- Stemberger RS. 1990. Food limitation, spination and reproduction in Brachionus calyciflorus. Limnol Oceanogr 85: 33–44. [CrossRef] [Google Scholar]
- Stemberger RS, Gilbert JJ. 1984. Spine development in the rotifer Keratella cochlearis: induction by cyclopoid copepods and Asplanchna. Freshwater Biol 14: 639–648. [CrossRef] [Google Scholar]
- Tollrian R, Duggen S, Weiss LC, Laforsch C, Kopp M. 2015. Density-dependent adjustment of inducible defenses. Sci Rep 5: 12736. [CrossRef] [PubMed] [Google Scholar]
- Xi YL, Wang J, Xie P, Huang XF. 2002. Morphological variation of Keratella cochlearis (Rotatoria) in a shallow, eutrophic subtropical Chinese lake. J Freshwater Ecol 17: 447–454. [CrossRef] [Google Scholar]
- Yin XW, Niu CJ. 2007. Polymorphism and morphotype transformations in the rotifer (Brachionus calyciflorus). Zool Res 28: 68–72. [Google Scholar]
- Yin XW, Niu CJ. 2008. Polymorphism in stem females and successive parthenogenetic generations in Brachionus calyciflorus Pallas. Aquat Ecol 42: 415–420. [CrossRef] [Google Scholar]
Cite this article as: Ge Y-L, Zhan R, Yu J-H, Xi Y-L, Ma J, Xu D-D. 2018. Effects of food concentration on the life table demography and morphology of three Keratella quadrata morphotypes. Ann. Limnol. - Int. J. Lim. 54: 16
All Tables
Life-table demography of offsprings produced by three K. quadrata morphotypes at four food concentrations (×106 cells·mL−1) (mean ± SE). 2PS: rotifer with two posterolateral spines; 1PS: rotifer with one posterolateral spine; 0PS: rotifer with none posterolateral spine. The different lowercase and capital letters represent significant differences among different concentrations and among three morphs, respectively. R0: net reproductive rate; rm: intrinsic rate of population growth; G0/G1/Gh: alternative measures of the generation time; PDH: population doubling time, AL: average lifespan.
Morphological characters of offsprings produced by three K. quadrata morphtypes at four food concentrations (×106 cells·mL−1) (mean ± SE). 2PS: rotifer with two posterolateral spine; 1PS: rotifer with one posterolateral spine; 0PS: rotifer with none posterolateral spine. The different lowercase and capital letters represent significant differences among different concentrations and among three morphs, respectively.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


