| Issue |
Int. J. Lim.
Volume 60, 2024
Special issue - Biology and Management of Coregonid Fishes
|
|
|---|---|---|
| Article Number | 10 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/limn/2024010 | |
| Published online | 29 July 2024 | |
Research article
Genetic origins of a resurging lake whitefish, Coregonus clupeaformis, population in the Detroit River, Laurentian Great Lakes
1
Freshwater Institute, Department of Fisheries & Oceans Canada, 501 University Crescent, Winnipeg, MB R3T 2N6, Canada
2
United States Geological Survey, Great Lakes Science Center, 1451 Green Road, Ann Arbor, MI 48105, USA
* Corresponding author: Wendylee.Stott@dfo-mpo.gc.ca
Received:
24
November
2023
Accepted:
10
June
2024
The Detroit River connects Lake Huron and Lake Erie of the Laurentian Great Lakes. The river once supported a substantial lake whitefish (Coregonus clupeaformis) fishery until the early 1900s, when habitat loss, pollution, and overfishing contributed to the collapse of the fishery and loss of spawning populations in the river. In the early 1970s, efforts were initiated to improve water and habitat quality, and in December 2005 a spawning male lake whitefish and viable eggs were collected; the first documented evidence of spawning since 1916. Researchers have tracked the spawning magnitude of the lake whitefish population in the Detroit River since 2005 by assessing the number of eggs deposited on egg mats. Genetic analysis of larval fish hatched from eggs collected in the field between 2005 and 2018 was used to determine the relative contributions of Lake Erie and Lake Huron to the resurging population. Over 80% of the hatched larvae had parents originating from Lake Erie in all the years sampled. The estimated number of full-sibling families sampled at Belle Isle was the same in 2010 and 2014 and varied between 2009 and 2016 at Fighting Island. The estimated number of lake whitefish parents at Fighting Island decreased in the most recent collections possibly due to loss of habitat on spawning reefs due to sedimentation. Our results provide additional evidence that restored spawning habitat in the Detroit River is again being used by lake whitefish and continued reproduction at these sites may improve the Great Lakes portfolio of ecological and genetic diversity.
Key words: Spawning habitat / restoration / individual assignment / effective number of breeders / Great Lakes
© W. Stott et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Rocky reefs and coarse substrates in freshwater lakes and streams provide habitat for aquatic species at different life history stages. For example, they are used by lithophilic fishes for spawning. The location and composition of reef habitat in rivers can make them prone to degradation caused by sedimentation related to pollution or water control, or reefs may be removed to clear passage for shipping lanes or for dam construction (Gunderson et al., 2008; Bennion and Manny, 2011). The St. Clair and Detroit rivers in the Laurentian Great Lakes are examples of rivers where fish spawning habitat was removed and degraded with a corresponding loss in fish abundance and species diversity (Fig. 1; Manny 2003), but more recently there have been environmental improvements resulting from regulation changes and targeted habitat restoration projects (Hartig et al., 2018, 2020).
The Detroit River supported a large lake whitefish (Coregonus clupeaformis) fishery until the early 1900s (Baldwin et al., 2009). Most of the fishing effort was located between the city of Detroit, MI, USA (42.3314 N, −83.0457 W), and the entry of the Detroit River into Lake Erie (Milner, 1874; Baldwin et al., 2009) when fishing occurred during the fall during spawning runs of lake whitefish and cisco (C. artedi). While the fishery had started to decline in the late 1800s due to overfishing and pollution (Milner, 1874), the construction of shipping channels also affected lake whitefish and other fish using the river for spawning (Smith, 1915). During the construction of shipping channels in the early 1900s, cobble and bedrock used as spawning habitat at Grassy Island (42.2236 N, −83.1342 W; Detroit River) were removed and water flows through the river were restricted or halted (Smith, 1915). The total area of habitat lost is unknown (Bennion and Manny, 2011), but the loss is one of the main reasons for the decline and limited recovery of several fish species in lakes Erie and Huron (Roseman et al., 2007; Manny et al., 2015). In the early 1970s, ecological restoration programs were initiated to improve water and habitat quality. In December 2005, a spawning male lake whitefish and viable eggs were collected; the first documented evidence of spawning since 1916 (Roseman et al., 2007). From 2004 to 2018, seven artificial spawning reefs were constructed near historical spawning areas for lake sturgeon (Acipenser fulvescens), walleye (Sander vitreus), and lake whitefish near Belle Isle, Fort Wayne (42.2974 N, −83.0933 W), Fighting Island, and Grassy Island in the Detroit River (Fig. 1; Manny et al., 2015). While there was little formal assessment of spawner abundance in the river prior to reef construction, annual indicators of egg deposition on reefs and the surrounding areas have been conducted since 2005 to document the continued use of the spawning habitat, suggesting that improvements in water quality have played a role in restoration of the river habitat as well as the addition of spawning habitat (Prichard et al., 2017; Fischer et al., 2018).
Little is known about what the genetic population structure of lake whitefish was historically in the Great Lakes. Multiple spawning grounds were documented at Belle Isle, Grassy Island, and the Livingstone Channel (42.0922 N, −83.1294 W) on the Detroit River (Goodyear et al., 1982a) and earlier reports suggest that lake whitefish once spawned in the St. Clair River (Milner, 1874). These spawning areas appear to have been used predominantly by lake whitefish migrating from the eastern basin of Lake Erie (Milner, 1874; Wright, 1955). Therefore, Detroit River lake whitefish may have been genetically distinct from Lake Huron, and the Lake Erie lake whitefish that used the river for spawning may have been part of a meta-population. Allozyme and microsatellite DNA data have been used to describe population structure in Lake Huron (Casselman et al., 1981; Stott et al., 2010, 2012). Single nucleotide polymorphisms (Euclide et al., 2022) and microsatellite DNA (Stott et al., 2013) data have been used to describe lake whitefish populations in Lake Erie. Several genetic populations were identified in Lake Huron; including the northern portion of the main basin, the North Channel, and Georgian Bay (Fig. 1; Casselman et al., 1981; Stott et al., 2010; Stott et al., 2012). Microsatellite DNA data identified little structure in contemporary or historical samples from Lake Erie but found that Lake Erie and Lake Huron were genetically distinct (Stott et al., 2013), while Euclide et al. (2022) found modest differentiation between samples from the western and eastern areas of Lake Erie.
While reef restoration and programs to improve water quality aim to create a self-sustaining and resilient system, biological factors are also important for successful restoration of native species. For example, genetic variation is a prerequisite for an evolutionary response to environmental changes encountered as a species enters a novel habitat (Levitan, 2004). The number of migrants moving into a restored habitat and the genetics of their source populations can have an impact on the probability that a restored habitat will be able to support a self-sustaining population. If the increased amount of spawning habitat is used by a small number of individuals, their fertilization rates may by impacted by reduced spawner density which in turn may have an impact on effective population size (Levitan, 2004). Therefore, monitoring resurging and restored populations by determining relative contributions of source populations and measuring spawning success of adults is important. In this study, we quantified the use restored reef habitat in the Detroit River by estimating the number of adults producing offspring at the reefs and determining the relative contributions of Lake Erie and Lake Huron populations using microsatellite DNA data. We hypothesized that most of the lake whitefish using the spawning grounds were from Lake Erie because it is consistent with previous observations of lake whitefish movement in the St. Clair and Detroit rivers (Milner, 1874; Wright, 1955).
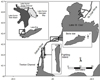 |
Fig. 1 Locations of sample collection sites in the Detroit River at Belle Isle and Fighting Island. Insets show the location of the reference populations in Lake Erie and Lake Huron, Lake Erie, Lake Huron-Main Basin, Lake Huron-Georgian Bay, and Lake Huron-North Channel (top right) used for the analysis of the samples. Arrows in insets show location of sampled spawning reefs. |
2 Materials and methods
2.1 Study area
The Detroit and St. Clair rivers and Lake St. Clair connect Lake Huron to Lake Erie. The St. Clair River starts at the outlet of Lake Huron and splits into several channels as it flows into Lake St. Clair, forming a delta. The Detroit River starts at the outlet of Lake St. Clair as a single channel and water flows around several islands before entering Lake Erie. Egg samples for this study were collected at the locations of the constructed spawning reefs at Fighting Island and Belle Isle (Fig. 1) and genetic samples from adults were collected from the Detroit River.
2.2 Sample collection
Fish eggs and adult fish were collected as part of an ongoing study to document the use of the constructed spawning reefs by several species (e.g., Roseman et al., 2011; Prichard et al., 2017; Fischer et al., 2018). The methods have been described elsewhere (e.g., Roseman et al., 2011; Fischer et al., 2018), but in brief, eggs were collected from gangs of egg mats placed on the river bottom at multiple sites. Sampling started in the fall when water temperatures dropped below 10 °C and ended when ice formed or poor weather made working on the river impossible. Sampling usually occurred between October and December each year. Each week, egg mats were retrieved, eggs were removed, counted, and transported to the US Geological Survey Great Lakes Science Center (Ann Arbor, MI USA) where they were incubated until they hatched. The species was determined using keys provided by Auer (1982) and then larvae were sacrificed and stored in 95% ethanol for genetic analysis (Tab. 1). Adult lake whitefish were collected during gillnet surveys conducted in the fall on the Detroit River.
Summary of lake whitefish (Coregonus clupeaformis) samples collected from sample sites in the Detroit River each year. Sample counts include adults and larvae hatched from eggs.
2.3 Genetic methods
Determining the origins of adult and eggs collected from the Detroit River required reference genotype data for lake whitefish that represented potential lake whitefish source populations for assignment tests. Lake whitefish representing potential source populations from lakes Erie and Huron were collected during or close to the spawning season from sites across both lakes. The details of the sources, collection methods, and observed population structure for these reference populations have been described elsewhere (Ebener et al., 2010; Stott et al., 2010, 2012, 2013).
We extracted DNA from the hatched lake whitefish larvae using the DNeasy protocol and reagents (Qiagen Inc., Hilden Germany) and determined the concentration of DNA using a DyNA Quant 200 (Hoefer, Inc., Holliston, MA, USA) fluorometer or an UV-Vis Cary spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA). Sample concentration was standardized to 100 ng μl−1 for amplification of microsatellite DNA loci via the polymerase chain reaction (PCR). Eight microsatellite DNA primers designed to amplify loci in lake whitefish (Bernatchez, 1996; Turgeon et al., 1999; Rogers et al., 2004), two primers designed for broad whitefish (C. nasus; Patton et al., 1997), and two primers designed for brook trout (Salvelinus fontinalis; Angers et al., 1995) were used. One of the two primers for each locus was labelled with a fluorescent dye. Each PCR reaction was carried out in a volume of 15 µl containing 0.25 to 0.75 U Taq polymerase, the manufacturer's (Promega Co., Madison, WI, USA) buffer at 1× concentration, 0.2 mM of each dNTP, 0.3–0.5 µM of each primer and 80 to 100 ng of template DNA. The PCR thermal profile was similar for all loci; only the annealing temperature changed depending on the primer set being amplified (Tab. 2). An initial denaturation step of 2 min at 94 °C was followed by 35 cycles of 1 min at 94 °C, 1 min at the annealing temperature, and 1 min extension at 72 °C, followed by one final extension for 5 min at 72 °C. Several loci were amplified in multiplex reactions to increase sample throughput efficiency (Tab. 2).
PCR products were prepared according to manufacturer's guidelines (Life Technologies, Carlsbad, CA, USA) for capillary electrophoresis. The PCR product was diluted with 9 µL of water. Formamide (10 µL) and 0.5 µL of a 400 base pair size standard (Applied Biosystems GeneScan 400HD ROX™ Size Standard, Life Technologies, Carlsbad, CA, USA) was added to 1 µL of the diluted PCR product. Samples were denatured for 4 min and then chilled for 3 min before they were loaded on the ABI 3130-AVANT Genetic Analyzer. Fragment size data were collected using the manufacturer's (Life Technologies, Carlsbad, CA, USA) software. Allele calls were assigned using the GeneMapper software (Life Technologies, Carlsbad, CA, USA) and then reviewed at least twice. Alleles for each fish were summarized into a genotype used for subsequent analyses. Any sample missing data at more than four loci was removed from the data set.
Loci used in analysis of lake whitefish (Coregonus clupeaformis), their PCR conditions, fluorescent labels used, and reference for each locus.
2.4 Data analysis
The morphological assignment of the hatched larval samples as lake whitefish was confirmed using genetics following the approach described by Stott et al. (2021) because cisco and bloater (C. hoyi) have also been captured in the Detroit River (Stott et al., 2021). We then used two approaches to determine the contributions of Lake Erie and Lake Huron lake whitefish populations to the samples collected from the Detroit River. Individual assignment tests were performed using GENECLASS (Piry et al., 2004) and Structure 2.3.4 (Pritchard et al., 2000) software packages. Both software packages use a Bayesian approach but use different algorithms and present the results in a different format. GENECLASS estimates the probability that an individual belongs to each reference population and assigns it to the most probable source while Structure estimates the proportional contribution of each reference population to an individual's genotype after grouping individuals into a user-defined number (K) of genetic clusters. Using two approaches will provide additional validation of results and allow us to analyze trends in different ways. Based on past analyses of the population structure of lake whitefish in lakes Erie and Huron (Stott et al., 2010, 2012, 2013), we divided the reference data set into four groups that represent the main sources of genetic variation: North Channel-Lake Huron, Main Basin-Lake Huron, Georgian Bay-Lake Huron, and Lake Erie (Fig. 1). These groups were used as reference populations for the analysis of larval samples using GENECLASS. Confidence in assignment to a reference population was also calculated using GENECLASS. A similar analysis was performed across two simulations in Structure by setting the number of assumed populations at K = 4 (the four populations described above) and then at K = 2 (Lake Erie vs. Lake Huron). At K = 2, we could also estimate the number of individuals with a parent from each lake, i.e., an inter-lake hybrid. An individual was classified as an inter-lake hybrid if its q-score for the cluster associated with Lake Erie was between 0.4 and 0.9. The Bayesian analysis was performed over 10 runs, each with a burn-in period of 100,000 iterations followed by 100,000 iterations. We selected the “admixture model” as our ancestry model and used the population information (USEPOPINFO = 1) to determine the origins of the samples from the Detroit River going back one generation (GENSBACK = 1). Individual membership coefficients (q) from each run were averaged and summarized by year and sampling location.
We examined the impact of temperature on the estimated number of hybrids each year by comparing the percent hybrids to the temperature in each lake. We performed a linear regression using the day of the year in the water temperature first fell below 8 °C and the percent of hybrids for each lake (NOAA, 2023) for each year from 2009 to 2018.
We used the program COLONY 2.0.6.8 (Wang, 2009; Jones and Wang, 2010) to estimate the number of families and effective number of breeding adults at each site for each year when sample sizes were greater than 20. COLONY uses a maximum likelihood method to assign samples to full and half-sibling families and to determine the minimum number of individuals that contributed to sampled genotypes (Wang, 2004; Wang and Santure, 2009). One hundred runs were performed for each set of samples and input parameters were: male and female polygamy without inbreeding and clonality, diploid and dioecious species, medium run using the full likelihood analysis method with high precision, allele frequencies were not updated, sib-ship sizes were scaled with no sibship size prior, all parameters involving maternity and paternity were unknown, and a genotyping error rate of 0.01. We ran the analysis for samples collected at each site in each year to look at changes in number of families and spawners using the site. We also ran samples collected at all sites in a year to look at patterns of reef use.
3 Results
Genotype data for 15 adult lake whitefish and 824 larvae from the Detroit River were collected for the analysis. All larvae were genetically confirmed to be lake whitefish. The samples were grouped by site and year. Samples from sites directly on or near the constructed reef habitat were grouped together because fish spawn on both sites (Fischer et al., 2018). The area where the samples were collected was not recorded for 128 samples, therefore analyses summarized by reef areas could only be made for 711 of the samples and the remaining samples were classified as coming from the Detroit River. Reference populations for the population assignment of the larvae included 2,534 lake whitefish from Lake Huron and 380 from Lake Erie. The reference data set was missing 0.9% of data across all loci; missing data by population ranged from 0.7% (Lake Huron-Main Basin) to 1.0% (Lake Erie) and by locus from 0% (CoclLav68) to 2.8% (CoclLav8). The larval and adult samples collected from the Detroit River were missing 5.3% of data across loci which ranged by locus from 0.6% (C2-157) to 13.2% (CoclLav52).
The accuracy of assignment of a fish to a source population was tested using GENECLASS and we found that 66% of the reference samples could be correctly assigned to their actual source population. The largest mis-assignments occurred among the Lake Huron populations, with Georgian Bay samples being classified as North Channel samples 18.2% of the time and as Main Basin samples 16.8% of the time. When we repeated the analysis using lake as a source (i.e., K = 2), samples were assigned correctly 91.0% of the time. In all years sampled, most of the hatched eggs were assigned to the group associated with Lake Erie, ranging from 76.5% of the samples in 2017 to 100% of the samples in 2005 and 2006 (Tab. 3). Samples assigned to Lake Huron populations were most frequently assigned to the Main Basin followed by Georgian Bay. Similar trends were observed when samples were categorized by collection area (Belle Isle and Fighting Island). Adults captured in the Detroit River (Belle Isle, Fighting Island, Trenton Channel) produced similar results, 11 of the 14 samples were classified as Lake Erie fish and three were assigned to the Georgian Bay population of Lake Huron (Tab. 1).
The Bayesian analysis using the Structure software was conducted using K = 4 to explore differences among genetic groups and at K=2 (Lake Huron and Lake Erie) to further explore differences among sites and years. Consistent with the GENECLASS results, more than 90% of the reference samples could be assigned correctly to their lake of origin and most samples were classified as having Lake Erie origins (Fig. 2). The number of inter-lake hybrids varied during the sampling period (Tab. 3) from a high of 23.6% in 2009 to a low of 0% in 2015, with fewer hybrids in later years (Fig. 2; Tab. 3). We tested if water temperature had an impact on the number of hybrids each year. Lake whitefish spawning begins when water temperature is between 5 and 8 °C in Lake Erie (Lawler, 1965; Edsall and Rottiers, 1976). We saw little evidence of a relationship between the proportion of hybrids and first day in fall and winter when water temperatures in Lake Huron (R2 = 0.14, F(1, 6) = 1.00, p = 0.355) and Lake Erie (R2 = 0.005, F(1, 6) = 0.033, p = 0.861) fell below 8 °C (Fig. 3).
The genotypes of lake whitefish collected from a reef area were used to estimate the number of individuals that spawned, the effective population size, and the number of families produced. Estimates of all metrics were similar for the two years of sampling at Belle Isle while all metrics increased for estimates from samples from Fighting Island until 2010 and then they appear to have declined (Tab. 4). We looked for evidence of use of both sites in 2010 and 2014 as those were the only years where >20 samples were collected from both sites. In 2010, 24 parents were estimated to have offspring at both sites with no full-sibling pairs and 24 half-sibling pairs. In 2014, the estimated number of parents was 26 and there was 1 full-sib and 26 half-sib pairs.
Summary of assignments (as percent of sample) lake whitefish (Coregonus clupeaformis) eggs collected from the Detroit River to populations in lakes Huron and Erie in each sampling year using GENECLASS and percent of inter-lake hybrids observed using results from Structure. N = sample size.
 |
Fig. 2 Genetic assignment of lake whitefish (Coregonus clupeaformis) eggs collected from the Detroit River between 2009 and 2018 calculated using the software package Structure. Each bar represents an individual fish and shows the proportion of its genotype associated with Lake Erie (black portion) and Lake Huron (white portion). |
 |
Fig. 3 Relationship between water temperatures of Lake Huron and Lake Erie and rate of inter-lake hybrids. Each graph shows the day of the year in the fall when the water temperature first falls below 8 °C for each year in the study versus the estimated proportion of inter-lake hybrids in each year. Each point is represented by the year in which data were collected (NOAA, 2023; https://coastwatch.glerl.noaa.gov/marobs/marobs.html). |
Lake whitefish (Coregonus clupeaformis) effective population size, number of adults, and number of full-sib pairs found at sites on the Detroit River estimated using genotypes from eggs collected in the Detroit River and COLONY. N = number of larvae included in simulation, Ne = effective population size, Nad = estimated number of breeding adults, Nfs = estimated number of full-sib pairs, CI = confidence interval.
4 Discussion
Detroit River reef restoration projects have re-established spawning habitat for several species, including lake whitefish (Fischer et al., 2018), thus providing greater availability and diversity of spawning habitat and, in turn, a healthier ecological portfolio. Analysis of microsatellite DNA data showed that lake whitefish from both Lake Huron and Lake Erie used the reefs to spawn between 2009 and 2018, although more fish from Lake Erie used them, consistent with our hypothesis. In the early period of our study, we observed an increase in the use of spawning areas in the Detroit River based on increases in numbers of parents observed at Fighting Island, however these numbers decreased in later years.
Lake whitefish appear to have resumed use of historical spawning areas in the Detroit River, despite the massive loss of spawning habitat early in the 20th century. Lake whitefish may have been migrating into the river throughout the twentieth century, but may have not been able to successfully spawn until recently. The last commercial landing of lake whitefish in the Detroit River was reported in 1925 (Roseman et al., 2007), but small numbers of lake whitefish were reported in other surveys (e.g., Wright, 1955). Lake whitefish eggs have been captured in areas throughout the Detroit River, but catch-per-unit effort is higher in areas near the constructed spawning reefs (Prichard et al., 2017); not surprising given reef placement was optimized to match the hydrodynamic preferences of the target species (lake sturgeon, lake whitefish, walleye) (Manny et al., 2015).
The observation that most lake whitefish using the reefs in the Detroit River are from Lake Erie is consistent with historical observations. Lake whitefish from northern and eastern parts of Lake Erie used the Detroit River for spawning and may have also used sites in the St. Clair River (Milner, 1874; Reighard, 1910; Smith, 1915). Lake whitefish migrated each year from the central and eastern basins of Lake Erie to reach spawning grounds in the western basin of Lake Erie and the Detroit and St. Clair rivers (Milner, 1874; Wright, 1955; Goodyear et al., 1982a,b). The microsatellite DNA loci we used could not reliably distinguish among populations in Lake Erie (Stott et al., 2013) to determine whether different populations used a specific reef area in the Detroit River. However, a recent analysis of Lake Erie population structure using SNPs (Euclide et al., 2022) identified two populations with high rates of gene flow between them; one associated with reefs in the southern part of the western basin and one composed of fish from the northwestern, central, and eastern basins of the lake. The central/eastern population identified by Euclide et al. (2022) may enter the Detroit River to spawn, while the southwestern population may use shoals and reefs that run from Port Clinton (41.5170 N, −82.9366 W) to South Bass Island (41.6481 N, −82.8262 W) and Kelleys Island (41.6060 N, −82.7080 W) as suggested by historical and recent lake whitefish spawning assessments (Amidon et al., 2021). Population structure in Lake Erie may have been more pronounced historically, due to differences in migratory behavior. Some fish from the central and eastern basins stayed in Lake Erie to spawn, while others made a longer migration into the Detroit River. This could be related to differences in migration patterns due to age and or genetics, for example, larger lake whitefish are more likely to move longer distances in Lake Huron (Li et al., 2017). Also, the chromosomal inversion that characterized the lake whitefish from the western Lake Erie population identified using genomic markers (Euclide et al., 2022) may explain a genetic basis for the differences in migratory behavior. Chromosomal inversions have been associated with life history differences in other species, including the migration and spawning behavior of Atlantic cod (Gadus morhua) (Puncher et al., 2019). Despite changes in availability in spawning habitat which may have increased gene flow between the two populations in Lake Erie or reduced the abundance of the northern population, the chromosomal inversion could have protected the region from recombination, helped maintain genetic differentiation between the two populations, and preserved the migratory behavior. Additional genotyping using genomics of lake whitefish captured in the Detroit River and fish from other lakes is needed to further explore this idea.
The proportion of lake whitefish from the study reefs with parents from Lake Huron was low and fairly constant through the sampling period. Historical reports indicate that lake whitefish from Lake Huron were less likely to move into the St. Clair River, although such movement was observed after heavy winds and storms originating from the north (Milner, 1874). Some of the fish could have been mis-assigned Lake Erie fish. However, the proportion of fish identified as Lake Huron in each year was usually greater than the assignment error rate, therefore some of those fish were likely from Lake Huron. Telemetry studies on walleye also noted limited movement between Lake Erie and Lake Huron. Walleye that migrated from Lake Erie would remain in the system longer than those from Lake Huron (Wang et al., 2007; Hayden et al., 2019). Future efforts to assess and include other lake whitefish spawning locations, such as those in the St. Clair River, could continue in order to monitor the contribution of Lake Huron and Lake Erie fish to this spawning population, and ultimately the Lake Erie adult lake whitefish population.
We observed more inter-lake hybrids in 2009 and 2010 than in subsequent years. However, the relative proportion of fish from Lake Huron remained relatively constant. Therefore something else may be related to the differences in the rate of hybridization by changing encounter probabilities between lake whitefish from each lake. For example, if the timing of when lake whitefish from each lake were using the reefs changed so they were not on reefs at the same time, then inter-lake hybrid rates would be lower in some years. If the timing of spawning was different in each lake, then there may not have been as much overlap in when lake whitefish from each lake moved into or through the Detroit River. However, we saw little evidence of a relationship between the proportion of hybrids and first day in fall and winter when water temperatures in Lake Huron and Lake Erie fell below 8 °C (Fig. 3). However, even if the use of the spawning reefs by lake whitefish from the two lakes did not change over time, changes in the dates when egg mats were set and retrieved may have influenced the results, so that in some years the egg mats were deployed at times when fish from both lakes were using the reefs while in other years they were not.
Lake whitefish spawn on the constructed reefs throughout the Detroit River. However, higher numbers of eggs were captured at sites directly upstream or downstream of the constructed reefs (DeBruyne et al., 2022a), typically in natural substrate with slightly smaller rock sizes than the reef rock (Manny et al., 2015). The numbers of families found at Fighting Island each year varied (73 on average) and were generally greater than the estimates for Belle Isle (19 and 15). These estimates should be treated as lower end estimates because all adults were likely not detected given the small number of samples and markers used. The numbers of families and effective number of breeder estimates were lower in recent collections, consistent with decreases in abundance possibly due to a decrease in sampling effort at sites within the reef areas where lake whitefish spawned and an increase in sedimentation at sites (Fischer et al., 2020). Changes are most notable at the Fighting Island reef area where the number of ‘off-reef’ sites sampled decreased from 12 in 2009 to 2 in 2015. The reduced numbers of sampling sites in some years (e.g., 2015 and 2016, Fighting Island, Tab. 4) may have also contributed to the lower number of spawners using COLONY because these estimates can be correlated with sample size (Wang and Santure, 2009; Hunter et al., 2020).
We found offspring from the same parents at different sites in 2010 and 2014. A similar result was found in a study of lake sturgeon on some of the same reefs (Hunter et al., 2020). They found eggs from the same parents at different sites and speculated that eggs may drift downstream after being spawned. We do not know if our results were also related to egg drift downstream (DeBruyne et al., 2022b) or if parents used more than one site; both explanations are possible in this fast-flowing river system.
Continued successful use of the Detroit River as a lake whitefish spawning area may help increase species genetic diversity and population stability in the Great Lakes. A healthy ‘portfolio’ of diversity in a species can provide stability at larger regional scales through a buffering effect that allows populations to respond to stresses that may have different impacts at different geographic scales (Schindler et al., 2010; DuFour et al., 2015). Effective habitat restoration projects provide a diversity in habitat that can help rebuild a healthy portfolio (Bouska et al., 2023) and possibly maintain multiple genetic populations. Multiple spawning stocks with restricted gene flow among them will result in greater overall genetic diversity relative to single populations of the same size (Whitlock and Barton, 1997). Our results provide additional evidence that restoration efforts in the Detroit River are providing spawning habitat for lake whitefish and that reef use is consistent with historical observations. Continued monitoring can help to determine whether spawners continue to use the reefs and to whether lake whitefish produced on the reefs survive.
Acknowledgements
This work was partially supported by funds from the Great Lakes Restoration Initiative and the Great Lakes Fish and Wildlife Restoration Act (Agreement #30181G020). Laboratory assistance came from Kära Davidson, Stacey Ireland, Melissa Kostich, Lynn Ogilvie, Baileigh Reed-Grimmett, and Jenny Sutherland. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. All sampling and handling of fish were carried out in accordance with guidelines for the care and use of fishes by the American Fisheries Society (Jenkins et al., 2014).
Author contributions statement
WLS and ER conceptualized the study. WLS coordinated genetic data collection and analyzed genetic data. WLS wrote the manuscript and revised it with input from all coauthors.
References
- Amidon Z, DeBruyne RL, Roseman EF, Mayer CM. 2021. Evidence of increased lake whitefish spawning distribution in Western Lake Erie. Adv Limnol 66: 163–172. [CrossRef] [Google Scholar]
- Angers B, Bernatchez L, Angers A, Desgroseillers L. 1995. Specific microsatellite loci for brook charr reveal strong population subdivision on a microgeographic scale. J Fish Biol 47: 177–185. [CrossRef] [Google Scholar]
- Auer NA (Ed.). 1982. Identification of Larval Fishes of the Great Lakes Basin with Emphasis on the Lake Michigan Drainage. Special Publication 82-3. Great Lakes Fishery Commission, Ann Arbor, Michigan. http://www.glfc.org/pubs/SpecialPubs/Sp82_3.zip [Google Scholar]
- Baldwin NA, Saalfeld RW, Dochoda MR, Buettner HJ, Eshenroder RL. 2009. Commercial fish production in the Great Lakes 1867–2006. Great Lakes Fishery Commission, Ann Arbor, Michigan. http://www.glfc.org/commercial/commerc.php [Google Scholar]
- Bouska KL, Healy BD, Moore MJ, Dunn CG, Spurgeon JJ, Paukert CP. 2023. Diverse portfolios: Investing in tributaries for restoration of large river fishes in the Anthropocene. Front Environ Sci 11: 426. [Google Scholar]
- Bennion DH, Manny BA. 2011. Construction of shipping channels in the Detroit River: history and environmental consequences. U.S. Geological Survey Scientific Investigation Report 2011–5122. https://pubs.usgs.gov/sir/2011/5122/pdf/sir 2011-5122. pdf [Google Scholar]
- Bernatchez L. 1996. Réseau de suivi environnemental du complexe La grande. Caractérisation génétique des formes naines et normales de grand corégone du réservoir caniapiscau et du lac Sérigny à l'aide de marqueurs microsatellites. Rapport présenté par l'université Laval à la vice-présidence Environement et Collectivités d'Hydro-Québec [Google Scholar]
- Casselman JM, Collins JJ, Crossman EJ, Ihssen PE, Spangler GR. 1981. Lake whitefish (Coregonus clupeaformis) stocks of the Ontario waters of Lake Huron. Can J Fish Aquat Sci 38: 1772–1789. [CrossRef] [Google Scholar]
- DeBruyne RL, Craig JM, Kennedy GW, Roseman EF. 2022a. Fish eggs collected in the St. Clair, Detroit, and St. Marys rivers, 2005–2022: U.S. Geological Survey data release, https://doi.org/10.5066/P9MMM8OT. [Google Scholar]
- DeBruyne RL, Tomczak MG, Schmidt BA, Bowser DA, Fischer JL, Kennedy GW, King NR, Mayer CM, Roseman EF. 2022b. Fish egg retention on egg mats in experimental flumes and targeted field gear egg collection in the Detroit River, 2015–2016: U.S. Geological Survey data release, https://doi.org/10.5066/P96H1ZBC. [Google Scholar]
- DuFour MR, May CJ, Roseman EF, Ludsin SA, Vandergoot CS, Pritt JJ, Fraker ME, Davis JJ, Tyson JT, Miner JG, Marschall EA, Mayer CM. 2015. Portfolio theory as a management tool to guide conservation and restoration of multi-stock fish populations. Ecosphere 6: 12. [CrossRef] [Google Scholar]
- Ebener MP, Brenden TO, Wright GM, Jones ML, Faisal M. 2010. Spatial and temporal distributions of Lake Whitefish spawning stocks in Northern lakes Michigan and Huron, 2003- 2008. J Great Lakes Res 36: 38–51. [CrossRef] [Google Scholar]
- Edsall TA, Rottiers DV. 1976. Temperature tolerance of young-of-the-year lake whitefish, Coregonus clupeaformis. J Fish Res Board Can 33: 177–180. [CrossRef] [Google Scholar]
- Euclide PT, Kraus RT, Cook A, Markham JL, Schmitt JD. 2022. Genome-wide genetic diversity may help identify fine-scale genetic structure among lake whitefish spawning groups in Lake Erie. J Great Lakes Res 48: 1298–1305. [CrossRef] [Google Scholar]
- Fischer JL, Pritt JJ, Roseman EF, Prichard CG, Craig JM, Kennedy GW, Manny BA. 2018. Lake sturgeon, lake whitefish, and walleye egg deposition patterns with response to fish spawning substrate restoration in the St. Clair-Detroit River system. Trans Am Fish Soc 147: 79–93. [CrossRef] [Google Scholar]
- Fischer JL, Roseman EF, Mayer C, Wills T. 2020. If you build it and they come, will they stay? Maturation of constructed fish spawning reefs in the St. Clair-Detroit River System. Ecol Eng 150: 105837. [CrossRef] [Google Scholar]
- Gunderson DR, Parma AM, Hilborn R, Cope JM, Fluharty DL, Miller ML, Vetter RD, Heppell SS, Greene HG. 2008. The challenge of managing nearshore rocky reef resources. Fisheries 33: 172–179. [CrossRef] [Google Scholar]
- Goodyear CD, Edsall TA, Ormsby-Dempsey DM, Moss GD, Polanski PE. 1982a. Atlas of spawning and nursery areas of Great Lakes fishes. Volume VIII: Detroit River. U.S. Fish and Wildlife Service FWS/OBS-82/52. https://ecos.fws.gov/ServCat/DownloadFile/221173 [Google Scholar]
- Goodyear CD, Edsall TA, Ormsby-Dempsey DM, Moss GD, Polanski PE. 1982b. Atlas of spawning and nursery areas of Great Lakes fishes. Volume IX: Lake Erie. U.S. Fish and Wildlife Service FWS/OBS-82/ 52. [Google Scholar]
- Hartig JH, Krantzberg G, Alsip P. 2020. Thirty-five years of restoring Great Lakes Areas of Concern: Gradual progress, hopeful future. J Great Lakes Res 46: 429–442. [CrossRef] [Google Scholar]
- Hartig JH, Sanders CE, Wyma RJH, Boase JC, Roseman EF. 2018. Habitat rehabilitation in the Detroit River Area of Concern. Aquatic Ecosyst Health Manag Soc 21: 458–469. [CrossRef] [Google Scholar]
- Hayden TA, Vandergoot CS, Fielder DG, Cooke SJ, Dettmers JM, Krueger CC. 2019. Telemetry reveals limited exchange of walleye between Lake Erie and Lake Huron: Movement of two populations through the Huron-Erie corridor. J Great Lakes Res 45: 1241–1250. [CrossRef] [Google Scholar]
- Hunter RD, Roseman EF, Sard NM, DeBruyne RL, Wang J, Scribner KT. 2020. Genetic family reconstruction characterizes Lake Sturgeon use of newly constructed spawning habitat and larval dispersal. Trans Am Fish Soc 149: 266–283. [CrossRef] [Google Scholar]
- Jenkins JA, Bart, Jr., HL, Bowker JD, Bowser PR, MacMillan JR, Nickum JG, Rachlin JW, Rose JD, Sorensen PW, Warkentine BE, Whitledge GW. 2014. Guidelines for use of fishes in research—revised and expanded. Fisheries 39: 415–416. [CrossRef] [Google Scholar]
- Jones OR, Wang J. 2010. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Res 10: 551–555. [CrossRef] [Google Scholar]
- Lawler GH. 1965. Fluctuations in the success of year-classes of whitefish populations with special reference to Lake Erie. J Fish Res Board Can 22: 1197–1227. [CrossRef] [Google Scholar]
- Levitan DR. 2004. Density-dependent sexual selection in external fertilizers: variance in male and female fertilization success along the continuum from sperm limitation to sexual conflict in the sea urchin Strongylocentrotus franciscanus. Am Nat 164: 298–309. [CrossRef] [PubMed] [Google Scholar]
- Li Y, Bence JR, Zhang Z, Ebener MP. 2017. Why do lake whitefish move long distances in Lake Huron? Bayesian variable selection of factors explaining fish movement distance. Fish Res 195: 169–179. [CrossRef] [Google Scholar]
- Manny BA. 2003. Setting priorities for conserving and rehabilitating Detroit River habitats, in: Honoring our Detroit River, caring for our home, edited by Hartig JH. Bloomfield Hills, Michigan: Cranbrook Institute of Science, pp. 121–139. [Google Scholar]
- Manny BA, Roseman EF, Kennedy G, Boase JC, Craig JM, Bennion DH, Read J, Vaccaro L, Chiotti J, Drouin R, Ellison R. 2015. A scientific basis for restoring fish spawning habitat in the St. Clair and Detroit rivers of the Laurentian Great Lakes. Rest Ecol 23: 149–156. [CrossRef] [Google Scholar]
- Milner JW. 1874. Report on the fisheries of the Great Lakes: The result of inquiries prosecuted in 1871 and 1872. Report of the US Commissioner of Fish and Fisheries for 1872 and 1873 2: 1–78. https://library.noaa.gov/Collections/Digital-Documents/Fish-Comm-Annual-Rep. [Google Scholar]
- NOAA. 2023. CoastWatch Great Lakes Node. Realtime Great Lakes Weather Data and Marine Observations from 2009 to 2018. Stations 45005 and W DD287. Great Lakes Environmental Research Laboratory https://coastwatch.glerl.noaa.gov/marobs/marobs.html Accessed 8/24/2023. [Google Scholar]
- Patton JC, Gallaway BJ, Fechhelm RG, Cronin MA. 1997. Genetic variation of microsatellite and mitochondrial DNA markers in broad whitefish (Coregonus nasus) in the Colville and Sagavanirktok rivers in northern Alaska. Can J Fish Aquat Sci 54: 1548–1556. [CrossRef] [Google Scholar]
- Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A. 2004. GENECLAS S2: a software for genetic assignment and first-generation migrant detection. J Hered 95: 536–539. [CrossRef] [PubMed] [Google Scholar]
- Prichard CG, Craig JM, Roseman EF, Fischer JL, Manny BA, Kennedy GW. 2017. Egg deposition by lithophilic-spawning fishes in the Detroit and St. Clair rivers, 2005–2014. U.S. Geological Survey Scientific Investigations Report 2017-5003. https://pubs.usgs.gov/publication/sir20175003 [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [Google Scholar]
- Puncher GN, Rowe S, Rose GA, Leblanc NM, Parent GJ, Wang Y, Pavey SA. 2019. Chromosomal inversions in the Atlantic cod genome: Implications for management of Canada's Northern cod stock. Fish Res 216: 29–40. [CrossRef] [Google Scholar]
- Reighard P. 1910. A plan for promoting the whitefish production of the Great Lakes. Bull U.S. Bur Fish 28: 643–684. [Google Scholar]
- Rogers SM, Marchand M-H., Bernatchez L. 2004. Isolation, characterization and cross-salmonid amplification of 31 microsatellite loci in lake whitefish (Coregonus clupeaformis, Mitchill). Mol Ecol Notes 4: 89–92. [CrossRef] [Google Scholar]
- Roseman EF, Kennedy GW, Boase J, Manny BA, Todd TN, Stott W. 2007. Evidence of Lake Whitefish spawning in the Detroit River: implications for habitat and population recovery. J Great Lakes Res 33: 397–406. [CrossRef] [Google Scholar]
- Roseman EF, Manny BA, Boase J, Child M, Kennedy GW, Craig J, Soper K, Drouin R. 2011. Lake Sturgeon response to a spawning reef constructed in the Detroit River. J Appl Ichthyol 27: 66–76. [CrossRef] [Google Scholar]
- Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465: 609–612. [CrossRef] [PubMed] [Google Scholar]
- Smith HM. 1915. Report of the Commissioner of Fisheries. Report of the U.S. Commissioner of Fisheries for the fiscal Year 1914 with Appendixes. Bureau of Fisheries, 1–81. [Google Scholar]
- Stott W, MacDougall T, Roseman EF, Lenart S, Chiotti J, Yule DL, Boase J. 2021. Genetic species identification of coregonines from juvenile assessment and commercial fisheries in Lake Erie, Detroit River and St. Clair River. Adv Limnol 66: 403–425. [CrossRef] [Google Scholar]
- Stott W, Ebener MP, Mohr L, Hartman T, Roseman EF, Johnson JE. 2013. Spatial and temporal genetic diversity of lake whitefish from northern Lake Huron and western Lake Erie. Adv Limnol 64: 205–222. [CrossRef] [Google Scholar]
- Stott W, Ebener MP, Mohr L, Schaeffer J, Roseman EF, Harford WJ, Johnson JE, Fietsch C-L. 2012. Genetic structure of lake whitefish populations in the northern main basin of Lake Huron. Adv Limnol 63: 241–260. [CrossRef] [Google Scholar]
- Stott W, VanDeHey JA, Sloss BL. 2010. Genetic diversity of lake whitefish in Lakes Michigan and Huron; sampling, standardization, and research priorities. J Great Lakes Res 36: 59–65. [CrossRef] [Google Scholar]
- Turgeon J, Estoup A, Bernatchez L. 1999. Species flock in the North American Great Lakes: molecular ecology of lake Nipigon ciscoes (Teleostei: coregonidae:Coregonus). Evolution 53: 1857–1871. [Google Scholar]
- Wang J. 2004. Sibship reconstruction from genetic data with typing errors. Genetics 166: 1963–1979. [CrossRef] [PubMed] [Google Scholar]
- Wang J. 2009. A new method for estimating effective population sizes from a single sample of multi-locus genotypes. Mol Ecol 18: 2148–2164. [CrossRef] [PubMed] [Google Scholar]
- Wang J, Santure AW. 2009. Parentage and sibship inference from multi-locus genotype data under polygamy. Genetics 181: 1579–1594. [CrossRef] [PubMed] [Google Scholar]
- Wang HY, Rutherford ES, Cook HA, Einhouse DW, Haas RC, Johnson TB, Kenyon R, Locke B, Turner MW. 2007. Movement of walleyes in Lakes Erie and St. Clair inferred from tag return and fisheries data. Trans Am Fish Soc 136: 539–551. [CrossRef] [Google Scholar]
- Whitlock MC, Barton NH. 1997. The effective size of a subdivided population. Genetics 146: 427–441. [CrossRef] [PubMed] [Google Scholar]
- Wright S, 1955. Limnological survey of western Lake Erie. U.S. Fish Wildl. Serv. Spec. Sci. Rep. Fish. 139: 341. https://fisherybulletin.nmfs.noaa.gov/content/limnological-survey-western-lake-erie [Google Scholar]
Cite this article as: Stott W, DeBruyne R, Roseman E. 2024. Genetic origins of a resurging lake whitefish, Coregonus clupeaformis, population in the Detroit River, Laurentian Great Lakes. Int. J. Lim. 60: 10:
All Tables
Summary of lake whitefish (Coregonus clupeaformis) samples collected from sample sites in the Detroit River each year. Sample counts include adults and larvae hatched from eggs.
Loci used in analysis of lake whitefish (Coregonus clupeaformis), their PCR conditions, fluorescent labels used, and reference for each locus.
Summary of assignments (as percent of sample) lake whitefish (Coregonus clupeaformis) eggs collected from the Detroit River to populations in lakes Huron and Erie in each sampling year using GENECLASS and percent of inter-lake hybrids observed using results from Structure. N = sample size.
Lake whitefish (Coregonus clupeaformis) effective population size, number of adults, and number of full-sib pairs found at sites on the Detroit River estimated using genotypes from eggs collected in the Detroit River and COLONY. N = number of larvae included in simulation, Ne = effective population size, Nad = estimated number of breeding adults, Nfs = estimated number of full-sib pairs, CI = confidence interval.
All Figures
 |
Fig. 1 Locations of sample collection sites in the Detroit River at Belle Isle and Fighting Island. Insets show the location of the reference populations in Lake Erie and Lake Huron, Lake Erie, Lake Huron-Main Basin, Lake Huron-Georgian Bay, and Lake Huron-North Channel (top right) used for the analysis of the samples. Arrows in insets show location of sampled spawning reefs. |
| In the text | |
 |
Fig. 2 Genetic assignment of lake whitefish (Coregonus clupeaformis) eggs collected from the Detroit River between 2009 and 2018 calculated using the software package Structure. Each bar represents an individual fish and shows the proportion of its genotype associated with Lake Erie (black portion) and Lake Huron (white portion). |
| In the text | |
 |
Fig. 3 Relationship between water temperatures of Lake Huron and Lake Erie and rate of inter-lake hybrids. Each graph shows the day of the year in the fall when the water temperature first falls below 8 °C for each year in the study versus the estimated proportion of inter-lake hybrids in each year. Each point is represented by the year in which data were collected (NOAA, 2023; https://coastwatch.glerl.noaa.gov/marobs/marobs.html). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


