| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 12 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/limn/2021010 | |
| Published online | 18 June 2021 | |
Research Article
Specific indicator invertebrates of urbanized habitats in tributary streams of the Luján River basin (Buenos Aires, Argentina)
1
Instituto de Ecología y Desarrollo Sustentable (INEDES), CONICET-UNLu, Rutas 5 y 7, Luján, Buenos Aires, Argentina
2
Departamento de Ciencias Naturales, CONICET, Universidad Nacional de Río Cuarto (UNRC), Córdoba, Argentina
3
Departamento de Ciencias Básicas, Universidad Nacional de Luján, Luján, Buenos Aires, Argentina
* Corresponding author: agusfa@outlook.com
Received:
8
October
2020
Accepted:
28
May
2021
The increase of urbanized areas produces disturbances in rivers and streams, and its widespread effects reduce water quality and threaten aquatic biota. The aim of this study was to analyze changes in the invertebrate communities of the Luján River basin (Buenos Aires Province, Argentina) in an urbanization gradient and to determine the specific indicator taxa of urbanized habitats. Nine sampling sites were selected in the Luján River basin, distributed along a land use gradient. At each sampling site physicochemical variables of water were recorded, and invertebrate samples were collected during four seasons. A Principal Component Analysis separated three groups of sites with different urbanization conditions: low (<15%), moderate (between 15% and 60%) and high urbanization (>60%). These groups showed differences in the concentration of dissolved oxygen, suspended particulate organic matter, and nitrates. The communities changed with urbanization conditions, with a simplification of the community composition and a decrease in richness towards the lower basin (moderate and high urbanization). The IndVal method found three indicator taxa for the low urbanization sites (Heleobia sp. (Cochliopidae), Uncancylus sp. (Ancylidae) and Callibaetis sp. (Baetidae)) and three for the moderate urbanization habitats (Nematoda, Hyalella curvispina (Hyalellidae) and Chironominae). These taxa were useful to identify different disturbance conditions due to urbanization, which makes them potential bioindicators in the diagnosis and monitoring of water quality in the Luján River basin.
Key words: Monitoring / Argentina / streams / land use / water quality
© EDP Sciences, 2021
1 Introduction
Streams and rivers represent the most threatened freshwater systems due to the combined effects of climate change, frequency of droughts, and floods (Milly et al., 2005; Xenopoulos et al., 2005), habitat fragmentation, and multiple anthropic stressors (Ormerod et al., 2010; Vörösmarty et al., 2010; Woodward et al., 2010). Some studies, such as those by Sponseller et al. (2001), Townsend et al. (2003) and Allan (2004) recognize that the increment of human actions at the landscape scale is one of the main threats to the ecological integrity of a basin. In consequence, understanding the relationships of species with the environment has been essential for the development of monitoring systems, and each water quality assessment will be more precise if the indicator species and habitat preferences are known (McGeoch and Chown, 1998; Tickner et al., 2000).
In recent years, the land use has been intensified in the Pampas region (Argentina) by agriculturization and urbanization processes. The advance of agriculture on environments of greater environmental fragility (Manuel-Navarrete et al., 2009; Jayawickreme et al., 2011) generates land degradation with a growing decrease in agroecosystem's sustainability, and the consequent increase of nutrients as a result of fertilization and pesticides runoff. This has impacted on the benthic invertebrates, increasing the density of those taxa that are more tolerant (Cortelezzi et al., 2015, 2019). In the early 2000, the Argentine agricultural sector changed its productive characteristics, with the adoption the feedlots and an increase in no-tillage practices associated with transgenic cultivars. These changes introduced a modification in the ecosystems that receive more inputs and generate more residues and waste into the environment (Viglizzo and Jobbágy, 2010), as well as changes in the biogeochemical cycles and soil erosion that increased the turbidity and the runoff of fertilizers and agrochemicals in the fluvial systems (de la Fuente and Suárez, 2008; Rosso and Fernández Cirelli, 2013; Amuchástegui et al., 2016). On the other hand, between 2001 and 2010 there was a population increase in the surrounding urban areas (which reached 97.2%) compared to rural areas (2.8%), with a higher number of urban centers (from 151 to 167) in a west-east direction towards the Buenos Aires Metropolitan Region. This significantly urbanized area is very close to the main rivers and streams, so these are affected by increased loads from wastewater, overcrowding, and settlements (Velázquez, 2013; Cazenave et al., 2014).
In the Luján River basin, similar human actions are observed throughout its area. Traditionally, most of the surface of the basin has been used for extensive and intensive agricultural-livestock exploitation, although in recent decades there has been an intensification of agricultural activities (Palomeque, 2007). In the last decade, a higher cattle concentration has been observed in sites close to streams, displaced by the cropping on areas that were not suitable for agriculture (Aizen et al., 2009; Medan et al., 2011). In the lower basin, urban and industrial use predominate. With the development of highways since the 1990s, urbanization has increased (e.g. gated communities, country clubs) in historically rural areas. Therefore, the process of alteration of riverbanks has spread not only on the main river but also in tributary streams (Bonvecchi and Zuleta, 2014). On the other hand, the extraordinary floods of the Luján River in recent years have affected large areas; their causes are partly natural because the river extends over its floodplain and rainfall has been more intense, and partly due to human actions, which increased the areas allocated for buildings.
It is known that urbanization produces frequent disturbances in rivers and streams, decreasing water quality and threatening aquatic biota (Prat, 1997; Paul and Meyer, 2001), and, compared to other land uses, its effects are more severe (Folke et al., 1997; Allan, 2004; Halstead et al., 2014). However, fluvial ecosystems can present a variety of responses to urbanization depending on the specific characteristics of each environment (Cerqueira et al., 2020). In response to the impacts, the aquatic invertebrate community structure is modified, causing the death of the most sensitive organisms that can lead to local extinctions and favoring the increase of those populations of organisms that are less sensitive (tolerant) to adverse conditions (Feld and Hering, 2007; Muralidharan et al., 2010; Ruiz-Picos et al., 2016). In general, it is difficult to identify a single stressor that produces a significant response in invertebrate communities in an urbanization setting; typically, several environmental factors are combined that act synergistically (Miserendino and Brand, 2009). In different regions of Argentina, invertebrate communities have been analyzed to evaluate anthropic effects (Rocha et al., 2020), but there were not studies of invertebrateś preferences with respect to urbanization in the Luján River basin.
Therefore, the aim of this study was to analyze changes in invertebrate communities of the Luján River basin along an urbanization gradient, and to determine the specific indicator taxa of urbanized habitats. We predicted that there would be significant differences in the community between habitats with different urbanization conditions.
2 Material and methods
2.1 Study area
The study was located in the Luján River basin that is situated in the northeast of Buenos Aires Province, in the Rolling Pampa, characterized by a temperate subtropical climate with a mean temperature of 25 °C in summer and 9.5 °C in winter. The mean annual rainfall is 950 mm with a maximum in spring and autumn (Andrade, 1986). The hydrological regime is fed by rainfall and groundwater in the middle and upper reaches, whereas in its lower reach it is influenced by the Paraná River fluctuations in which it empties (Fidalgo, 1983; Andrade, 1986). Rainfall presents great spatial and temporal variability in the basin, and sometimes it is possible to observe large differences in the amount of water falling between nearby regions (Denegri et al., 2014).
The basin has a total area of 3761 km2 (Buzai and Principi, 2017), presents the highest drainage in the province (0.16 km−2) and has a low slope (1 m km−1) (Sala et al., 1983). The relief is predominantly uniform; it is a plain of the Pampean sedimentary type in the Buenos Aires sector, and an alluvial plain still in formation process in the Paraná delta (Andrade, 1986). According to the Instituto Nacional del Agua (INA, 2007) the basin can be divided into three sections: upper basin, middle basin and lower basin.
In this study, nine sampling sites were selected in tributary streams of the Luján River, distributed throughout the basin along a land use gradient (Fig. 1). The proportion of the different land uses (urban, vegetation and rural) was determined in a buffer area of a 2 km radius around each sampling point using Quantum GIS 3.4.9 software (Quantum GIS Geographic Information System, 2017). A supervised classification was carried out and the program classified pixels through the minimum distance method. The vegetation recorded by satellite images corresponded mostly to urban land use, this was checked through field work.
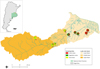 |
Fig. 1 Location of the nine study sites in the upper, middle, and lower basin of the Luján River (Buenos Aires province). |
2.2 Sampling of water physical and chemical variables and the invertebrate community
At each sampling site, richness and coverage (%) of macrophytes (emergent, floating and submerged) and observations of visible evidence of pollution sources (urban or road discharge, industrial discharge and agricultural discharge), water flow and watercolor were recorded in four periods (July 2017, November 2017, February 2018 and May 2018). In addition, nitrates and phosphates were estimated using a colorimetric method (Strickland and Parsons, 1968; Law al and Adeloju, 2013), turbidity with a calibrated Secchi tube (Dahlgren et al., 2004), conductivity and temperature were determined with a portable probe HI 9030 (HANNA) and pH was measured with a AD11 (Adwa) probe. Water samples were collected for the determination of dissolved oxygen (DO) by the Winkler method using the iodometric test with the azide modification; Biological Oxygen Demand (BOD5) by the dark incubation method for five days; Chemical Oxygen Demand (COD) by digestion with potassium dichromate; and Suspended Particulate Organic Matter (SPOM) and Suspended Particulate Inorganic Matter (SPIM) by a gravimetric method based on the retention of solid particles in a GF/C filter (APHA, 2012).
The collection of the invertebrate samples was carried out through a multi-habitat sampling (Barbour et al., 1999) with a D-net of 400 μm pore size; it was dragged countercurrent by gentle blows on the bed covering the environmental heterogeneity and walking upstream for 10 minutes. The samples were fixed in 4% formaldehyde for 48 h for subsequent taxonomic identification and preserved in 70% ethanol. Prior to identification, erythrosine drops were added to stain the organisms for better visualization, avoiding loss of biological material. Identification was performed under binocular and optical stereoscopic microscope. The taxonomic determinations were made to family, subfamily or genus for Cnidaria, Mollusca, Insecta, and Crustacea (Rumi et al., 2008; Lozano et al., 2009; Webb and Suter, 2011; Prat and Rieradevall, 2014). The determinations of the other taxa as Platyhelminthes and Annelida were made to class or subclass levels (Domínguez and Fernández, 2009). Then the taxa were classified according to functional feeding groups (FFGs), following Cummins and Klug (1979) and Cummins et al., (2005), into gatherers collectors (GC), which ingest sediment or gather loose particles in depositional areas; filterers collectors (FC), which suspension feed on particles from the water column with specialized anatomical structures; shredders (SH), which chew conditioned litter or live vascular plant tissue or gouge wood; scrapers (SC), that graze rocks and wood surfaces or stems of rooted aquatic plants, and predators (PR), which capture and engulf prey or tissue, and ingest body fluids.
2.3 Data analysis
Ordination of samples was performed using principal component analysis (PCA) based on the average of the physical, chemical and urbanization (%) data for the four periods. Statistical assumptions of homogeneity (Levene) and normality (Shapiro-Wilk) were assessed. Variables, except pH values, were log (x + 1) transformed to stabilize variances and normalize the data sets. Based on the PCA results, the first axis of the reduced space was chosen to represent the three conditions of urbanization.
The relationship between the disturbance gradient and functional feeding groups of the community was evaluated with Spearman rank correlations between the PCA axis and diversity index, percent gatherers collectors, percent filterers collectors, percent shredders, percent scrapers, and percent predators.
From the PCA, rank abundance curves were constructed for the different urbanization conditions, with the more abundant taxa (relative abundance >1%), which were ordered from the dominant to the less abundant. The organisms were used to observe the compositional and structural differences among the different urbanization conditions. These curves, in combination with species identity, can provide insight into specific patterns of species diversity, dominance, rarity, and composition (e.g., Vidaurre et al., 2006; Cultid-Medina and Escobar, 2016).
To evaluate changes in invertebrate community composition and abundance between the different urbanization conditions a Non-Metric Multidimensional Scaling (NMDS) was performed with a distance matrix constructed with the Bray-Curtis coefficient, and to test the hypothesis that the communities differed, an analysis of similarity (ANOSIM) was performed. Abundance data were transformed log10 (Y + 1) for the ordinations, and they were carried out using the vegan package of the statistical software R version 3.6.2. (R Development Core Team, 2019).
Indicator taxa were obtained using the IndVal method. The first step in this approach is obtaining a classification of sample units. The second step is to identify indicator taxa corresponding to the groups of the site typology. Indicator taxa are defined as the most characteristic taxa of each group, found mostly in a single group of the typology and present in the majority of the sites belonging to that group (Dufrêne and Legendre, 1997). These indicator taxa are those that are always present at certain sites and never occur in others; its indicator value ranges from 0 to 100, and the latter corresponds to a perfect indication. The significance of the indicator value for each taxa was tested using Monte Carlo test with 1000 permutations. Taxa with significant indicator values (p < 0.05) and higher than 65% (van Rensburg et al., 1999) were considered characteristic indicator taxa of the ecological condition in question. The IndVal method was carried out using PC-ORD version 5.0 (McCune and Mefford, 1999).
3 Results
The PCA performed with the average of the abiotic variables for the four periods explained 73% of the variance with the first two factors. The first axis was directly correlated positively with DO and negatively with nitrates and SPOM (50.9% variance explained), while the second axis was directly correlated with conductivity and pH (Fig. 2a). Through PCA, we identified three groups of sites (Fig. 2b). The groups defined by this arrangement were maintained throughout the data analysis in order to evaluate differences in the invertebrate community. These groups represent a gradient of the basin with different anthropic pressure in relation to urbanization: low (urbanization <15%), moderate (urbanization between 15% and 60%) and high (urbanization >60%) (Tab. 1). The first group (low urbanization), composed by the BA2, BA26 and BA4 sites, presented the highest levels of DO and low levels of nitrates and SPOM, and the second group (moderate urbanization), composed by BA6, BA9, BA12 and BA8, had a high phosphate content. The third group (high urbanization), that includes BA7a and BA7b, presented high concentration of nutrients, turbidity, SPOM and oxygen demands (COD) (Appendix 1).
Observations in the local surroundings of the sampled sites showed a predominance of urban and road discharge, as main pollution source, and brown watercolor, mainly (Tab. 1). BA4, BA2 and BA26 (low urbanization sites) had higher richness and coverage of the three biological groups of macrophyte. BA12, BA8, BA26, BA9, BA2, BA6 and BA4 sites were characterized by silt-clay substrate, mainly. BA2 and BA9 also presented pebbles, and BA6 and BA4 sand. BA7a and BA7b were the two different sites because their channels are covered with cement.
A total of 94 taxa were found in the basin, 80 of them were present in BA2, BA26 and BA4 (low urbanization), 71 taxa in BA8, BA9, BA12 and BA6 (moderate urbanization), and 14 taxa in BA7b and BA7a (high urbanization). The sites with the highest taxonomic richness were BA26 and BA2, with 62 and 46 taxa respectively (Appendix 2). Scraper abundance was higher at the sites with low urbanization, while gatherer collector abundance was higher at the sites with high urbanization (BA7b and BA7a) (Fig. 3). Shredder abundance was higher at the sites with moderate urbanization. H. curvispina, the most abundant shredder taxon, decreased from 68% at BA8 to 3% at BA2. Filterer collectors represented a minor component of the invertebrate communities, amounting to roughly 12% of the total abundance at the sites with high urbanization, while representing 1% and 2% at the sites with moderate and low urbanization, respectively. PCA axis 2 was correlated negatively with scraper percent (R = −0.83), but positively correlated with shredder percent (R= 0.72), while the axis 1 was positively correlated with diversity (R = 0.73).
In the low urbanization sites Heleobia sp. (Gastropoda), Caenis sp. and Callibaetis sp. (Ephemeroptera) (Fig. 4) presented high abundance; these are considered organisms sensitive to contamination with organic matter, like as Americabaetis (present in low proportion). Nematodes, oligochaetes, tardigrades and chironomids (Chironominae) were the more abundant organisms in sites with moderate and high urbanization. H. curvispina presented a great abundance along the gradient (except in BA7b), being predominant in the sites BA8 and BA6 (moderate urbanization).
Likewise, significant differences were found between the invertebrate communities present in the sites with different urbanization conditions (ANOSIM, R global = 0.5006; p = 0.001), as seen in the NMDS diagram (stress = 0.191, Fig. 5). Heleobia sp. and Pomacea sp. (Gastropoda), Callibaetis sp., Caenis sp., and Americabaetis sp. (Ephemeroptera), and Orthocladiinae (Chironomidae) were all associated with the sites of low urbanization, located in the upper right quadrant (Fig. 5); Acari, H. curvispina and Turbellaria were associated with the sites of moderate urbanization; and Chironominae, Tardigrada, Nematoda, Psychodidae and Culicidae were associated with the sites of moderate and high urbanization, located to the left of the first axis. Most of these sites are in the middle and lower basin.
The high urbanization sites are characterized by three indicator taxa, of which Heleobia sp. presented the highest value (99.0%). The moderate urbanization sites are characterized by three indicator taxa (Tab. 2), of which Nematoda presented the highest value (85.30%). For the high urbanization sites, no indicator taxa were found.
 |
Fig. 2 Ordination plot according to the Principal Component Analysis of (a) environmental variables and (b) study sites. Variables: Nitrates (mg L−1), Phosphates (mg L−1), Turbidity (NTU), pH, Water Temperature (°C), DO (mg L−1), COD (mg O2.L−1), BOD5 (mg O2.L−1), SPOM (g L−1), SPIM (g L−1), Conductivity (μS cm−1), Urbanization (%). Sites: red crosses for high urbanization sites, blue circle for moderate urbanization sites and green triangles for low urbanization sites. |
Locations and environmental features of the study sites. The different land uses were determined with Quantum GIS 3.4.9.
 |
Fig. 3 Percentages of invertebrate functional feeding groups (FFG) sampled from the study sites. References: SH: Shredder; FC: Collector-Filterer; SC: Scraper; GC: Gatherer-Collector; PR: Predator. |
 |
Fig. 4 Rank-abundance curve of the three urbanization conditions. |
 |
Fig. 5 Diagram of Non-metric multidimensional scaling (NMDS) of the invertebrate communities in three urbanization conditions. |
Indicator values (IndVals) for invertebrate taxa of the sites with different condition of urbanization. Monte Carlo test was used to assess the significance of each taxa as an indicator for urbanization. Only taxa with significant indicator values (p < 0.05) are listed.
4 Discussion
The aim of this study was to explore the effect of urbanization on invertebrate communities in tributary streams of the Luján River basin distributed in the upper, middle and lower basin, that the results show that Nematoda, H. curvispina and Chironominae are characteristic of the sites with moderate urbanization (between 15% and 60%), while Heleobia sp., Uncancylus sp. and Callibaetis sp. of the sites with low urbanization (<15%). The sites of the upper basin (low urbanization) presented the highest values of macroinvertebrate richness; these sites were also characterized by presenting greater coverage and richness of aquatic vegetation, high levels of dissolved oxygen and low concentrations of nitrates. In the moderate and high urbanization sites, there was a 22.7% decrease in taxa compared to the low urbanization ones, with a predominance of nematodes. Cortelezzi et al. (2019) found similar results in the richness of taxa when they analyzed the effect of an urbanization gradient in streams of a basin in Tandil (Buenos Aires). Likewise, several authors have reported that the invertebrate communities of urban streams are generally characterized by having a lower diversity of species, less trophic complexity, altered food webs, a modified community composition, and being numerically dominated by a few species of tolerant taxa such as oligochaetes and chironomids (Aguiar et al., 2002; Walsh et al., 2005; Miserendino et al., 2008). Taxonomic changes in urbanized areas could be explained by the modification of the resources that enter and predominate in the fluvial system, as well as by both a simplification and a homogenization of habitats.
Furthermore, the absence or less richness and coverage of aquatic vegetation in the more urbanized sites, in the lower basin, could affect the distribution of taxa. These variables produce an environmental gradient that is negatively related to urbanization (Monk et al., 2019). Urbanization is associated with loss and simplification of vegetative structure (McKinney, 2002), and remaining habitat (Marzluff and Ewing, 2001). It is known that the absence of vegetation in the impacted sites is the first cause of the deterioration of the quality of the habitat since it affects its heterogeneity and, consequently, its quality (Cortelezzi et al., 2019). In addition, macrophytes show a structuring role in streams with low slopes, high levels of nutrients and high irradiance, regulating and modifying the physical-chemical and biological characteristics of streams (Champion and Tanner, 2000), rather than the different types and sizes of substrata (Casset et al., 2001; Ferreiro et al., 2011; Cortelezzi et al., 2013; Cochero et al., 2016).
The urbanization gradient along the studied basin, from upper to lower parts, determined the chemical composition of the water. Furthermore, Giorgi et al. (2000) have observed that the physical and chemical variables showed an increasing human impact towards the lower basin. In general, there is a set of parameters such as dissolved oxygen, BOD5, nitrates and, to a lesser extent, SPOM, conductivity and turbidity, which more accurately reflect sites with impacts or alterations in water quality (Hwang et al., 2016). On the contrary, other variables such as pH and SPIM, are more related to the natural conditions of the basin, being less altered by anthropic changes in water quality. Our records coincide with the ranges of values reported by other authors in previous studies in the Luján River basin (Feijoó et al., 1999; Giorgi et al., 1999; O'Farrell et al., 2002; Pizarro and Alemanni, 2005; Lombardo et al., 2010; Sánchez Caro et al., 2012; Castañé et al., 2015).
In our study, the analysis of the physicochemical variables allowed us to define three groups of sites according to some parameters. The highest nitrate concentrations were recorded in May in the high urbanization sites: BA7a and BA7b (in Claro stream), whose margins are covered with cement, which would cause a decrease in the vertical and lateral hydrological connectivities, and a reduction of habitat heterogeneity (Sánchez-Pérez and Trémolières, 2003; Chelsea Nagy et al., 2011). The specific contributions they receive from inefficient wastewater treatment plants would increase nutrient concentrations (Meyer et al., 2005). On the other hand, these sites are the least vegetated, and do not have natural riparian vegetation that can counteract the diffuse contributions of nutrients (Naiman and Décamps, 1997; Sabater et al., 2003; Taylor et al., 2004). Moreover, although in all the studied sites habitat alteration was observed due to urban discharge, this was increasing along the urbanization gradient, together with the contribution of industrial discharges (sites BA12, BA7a and BA7b).
The high and moderate urbanization sites presented a high relative abundance of gatherer collectors, predators and shredders, which was significantly higher than in the low urbanization sites. Similar results have found Tagliaferro et al., (2020) in urban streams of the Pampean region. H. curvispina is the most abundant shredder taxa, as was also reported by Solis et al., (2019) in the coastal strip of the Río de la Plata (Buenos Aires). We observed that scraper was the best represented group in the low urbanization sites, which also agrees with Solis et al. (2019), and Moore and Palmer (2005), that found more abundance of this group in agricultural sites. However, a clear pattern of significant differences was not found between the different urbanization conditions, as observed by Rodrigues Capitulo et al. (2020) in streams in the Pampean area.
In high urbanization sites we did not find indicator taxa through the IndVal method, this could be due to the low abundance of individuals collected in these sites. The moderate urbanization sites were characterized by Nematoda, H. curvispina and Chironominae. These taxa are commonly found in deteriorated environments (Rosenberg and Resh, 1993), therefore our results suggest the degradation of the aquatic environment in the moderate urbanization sites. The presence of nematodes is generally associated with water courses with fine and superficial sediments, with abundant organic matter and colonized by bacteria and other organisms (Wetzel, 2001; Ezcurra de Drago et al., 2007). Moreover, Nematoda is considered a tolerant taxon (Bechara, 1996), since it showed great tolerance to high levels of conductivity and concentration of heavy metals (Paggi et al., 2006; Scheibler and Ciocco, 2011). Regarding H. curvispina, Grapentine and Rosenberg (1992) and Colla and César (2019), have observed that it prefers waters with high pH, but it occurred in relatively acidic waters in our study. On the other hand, Miserendino (2001), Moya et al. (2009) and Colla and César (2019) have observed this species in waters with low conductivity values (20–309 µS cm−1, 18.1–248 µS cm−1 and 15–447 µS cm−1, respectively), ranges much lower than those observed in our work (149–1900 µS cm−1), because the conductivity is higher in the Luján River basin due to its geological origin. These results express the plasticity of the species to the presence of ions. Chironominae is generally associated with waters of low pH (Medina and Paggi, 2004; Medina et al., 2008), and several authors have reported the presence of species of this subfamily associated with sites of low water quality (Wantzen et al., 2016; Serra et al., 2017; Molineri et al., 2020).
This study showed that many taxa were lost in moderate and high urbanization sites, such as Heleobia sp., Uncancylus sp. and Callibaetis sp., which were characteristic of low urbanization sites. These taxa play a key role in the aquatic ecosystem, and their loss represents the absence of organisms of great ecological value for the area and for global biodiversity in general (Nieto et al., 2017; Cortelezzi et al., 2019). Heleobia sp. is an opportunistic species (Tietze, 2011), which is associated with pH values close to 8 (Tietze et al., 2019), more basic than in the sites studied in this work, and with plasticity for salinity (Cazzaniga, 2011; Tietze et al., 2019). The distribution of Uncancylus sp. is related to DO, pH, temperature (César et al., 2012; Martín and Díaz, 2012) in ranges similar to ours, and to the presence of macrophytes (Rodrigues Capitulo et al., 2010). Callibaetis sp. is associated with high DO values (de Melo et al., 2002), and low conductivity and nutrients (Ocón and Rodrígues Capítulo, 2004), as those recorded in this study. Besides, Firmiano et al., (2017) have reported that it presents sensitivity to disturbance of the riparian zone, as well as to the increase of fine sediments in the stream bed due to increased urbanization in the basin; these observations would support the loss of Callibaetis sp. in more urbanized environments. According to Cortelezzi et al. (2013), when degradation is low, there are changes in the structure of the ecosystem and some sensitive species could disappear. However, the complete extinction of sensitive organisms in urban areas, together with the decrease in the total number of taxa and the increase in the abundance of tolerant taxa, indicates a severe degradation of the ecosystem.
Our results clearly show that the indicator taxa for low and moderate urbanization sites are useful to identify different disturbance conditions in the Luján River basin, which makes them useful bioindicators in the diagnosis and monitoring of water quality in the basin. This method not only improves the efficiency of bioindication systems but is likely to increase their successful adoption in management activities, also as it works with an extensive data set, the results are not biased by seasonal changes in taxa abundances (Davis, 1997). The condition of the river can be assessed using only the presence of aquatic invertebrates (Barbour et al., 1999), but a better understanding of the ecological preferences of taxa is needed. Our study, in particular, identifies indicator taxa and shows the ecological preferences of some characteristic taxa of the Luján River basin, because we worked with a representative data set, which included the full spectrum of seasons.
The information obtained and the conclusions drawn from this study could contribute to the management of the Luján River basin and to help policymakers to make sound, informed political decisions aimed at controlling pollution and the use of resources in the basin.
Acknowledgements
We sincerely acknowledge to Romina Príncipe for their constructive suggestions to improve the manuscript and Carlos Coviella for their critical reading. We thank Universidad Nacional de Luján personnel for their access and field assistance to some of the study sites. Also, we thank Agustina Silvera for their assistance in the field and lab work. This work was supported by the project PI4 (DISPCD-CBLUJ:0000503-18), Universidad Nacional de Luján and the HSBC Water Programme (FreshWater Watch project, Earthwatch Institute).
Appendix 1
Mean values of physical and chemical parameters in each study site in four periods. In parentheses are the standard deviation values.
Appendix 2
Mean abundance for four periods (standard deviation between brackets) and Functional Feeding Group (FFG) of invertebrate taxa at each site. References: GC (gatherers collectors), FC (filterers collectors), SH (shredders), SC (scrapers) and PR (predators).
References
- Aguiar EC, Ferreira MT, Pinto P. 2002. Relative influence of environmental variables on macroinvertebrate assemblages from an Iberian basin. J North Am Benth Soc 21: 43–53. [Google Scholar]
- Aizen M, Garibaldi L, Dondo M. 2009. Expansión de la soja y diversidad de la agricultura argentina. Ecol Austral 19: 45–54. [Google Scholar]
- Allan JD. 2004. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst 35: 257–284. [CrossRef] [Google Scholar]
- Amuchástegui G, di Franco L, Feijoó C. 2016. Catchment morphometric characteristics, land use and water chemistry in Pampean streams: a regional approach. Hydrobiologia 767: 65–79. [Google Scholar]
- Andrade MI. 1986. Factores de deterioro ambiental en la cuenca del Río Luján. Contribución del Instituto de Geografía, Facultad de Filosofía y Letras (UBA), Buenos Aires, p. 224. [Google Scholar]
- American Public Health Association (APHA), American Water Works Association, Water Environment Federation. 2012. Standard Methods for the Examination of Water and Wastewater. Washington: American Public Health Association, p. 541. [Google Scholar]
- Barbour MT, Gerritsen J, Snyder BD, Stribling JB. 1999. Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. In: Monitoring and Assessing Water Quality. Appendix B: (Part I), EPA 841–B–99–002, 2nd edn. Washington, DC: U.S. Environmental Protection Agency, Office of Water. [Google Scholar]
- Bechara JA. 1996. The relative importance of water quality, sediment composition and floating vegetation in explaining the macrobenthic community structure of floodplain lakes (Paraná River, Argentina). Hydrobiologia 333: 95–109. [Google Scholar]
- Bonvecchi VE, Zuleta GA. 2014. Degradación y pérdida de áreas ribereñas en el partido de Luján. In: Carballo CT and Goldberg S (eds.), Comunidad e información ambiental del riesgo. Las inundaciones y el río Luján. Argentina: Editorial Dunken, pp. 95– 108. [Google Scholar]
- Buzai GD, Principi N. 2017. Identificación de áreas de potencial conflicto entre usos del suelo en la cuenca del Río Luján, Argentina. Rev Geogr Am Cent 59: 91–124. [Google Scholar]
- Casset MA, Momo FR, Giorgi AD. 2001. Dinámica poblacional de dos especies de anfípodos y su relación con la vegetación acuática en un microambiente de la cuenca del río Luján (Argentina). Ecol Austral 11: 79–85. [Google Scholar]
- Castañé PM, Sánchez-Caro A, Salibián A. 2015. Water quality of the Luján river, a lowland watercourse near the metropolitan area of Buenos Aires (Argentina). Environ Monit Assess 187: 645. [Google Scholar]
- Cazenave J, Bacchetta C, Rossi A, Ale A, Campana M, Parma MJ. 2014. Deleterious effects of wastewater on the health status of fish: a field caging study. Ecol Indic 38: 104–112. [Google Scholar]
- Cazzaniga NJ. 2011. Notas autoecológicas sobre Heleobia parchappii. In: Cazzaniga NJ (ed.), El género Heleobia en América del Sur. Amici Molluscarum special number. Sociedad Malacológica de Chile (SMACH) pp. 26–28. [Google Scholar]
- Cerqueira TC, Mendonça RL, Gomes RL, de Jesus RM, da Silva DML. 2020. Effects of urbanization on water quality in a watershed in northeastern Brazil. Environ Monit Assess 192: 1–17. [Google Scholar]
- César II, Martín SM, Rumi A, Tassara M. 2012. Mollusks (Gastropoda and Bivalvia) of the Multiple-Use Reserve Martín García Island, Río de la Plata River: biodiversity and ecology. Braz J Biol 72: 121–130. [Google Scholar]
- Champion PD, Tanner CC, 2000. Seasonality of macrophytes and interaction with flow in a New Zealand lowland stream. Hydrobiologia 441: 1–12. [CrossRef] [Google Scholar]
- Chelsea Nagy R, Graeme Lockaby G, Kalin L, Anderson C. 2011. Effects of urbanization on stream hydrology and water quality: the Florida Gulf Coast. Hydrol Process 26: 2019–2030. [Google Scholar]
- Cochero J, Cortelezzi A, Tarda AS, Gómez N. 2016. An index to evaluate the fluvial habitat degradation in lowland urban streams. Ecol Indic 71: 134–144. [Google Scholar]
- Colla MF, César II. 2019. Ecological aspects of natural populations of Hyalella pampeana (Crustacea, Amphipoda, Hyalellidae) from the Natural Reserve Island of Martín García (Río de La Plata, Argentina). Ann Acad Bras Cienc 91: e20170928. [Google Scholar]
- Cortelezzi A, Sierra MV, Gómez N, Marinelli C, Rodrigues Capítulo A. 2013. Macrophytes, epipelic biofilm, and invertebrates as biotic indicators of physical habitat degradation of lowland streams (Argentina). Environ Monit Assess 185: 5801–5815. [CrossRef] [PubMed] [Google Scholar]
- Cortelezzi A, Ocón C, López van Oosterom MV, Cepeda R, Rodrigues Capítulo A. 2015. Nutrient enrichment effect on macroinvertebrates in a lowland stream of Argentina. Iheringia Sér Zool 105: 228–234. [Google Scholar]
- Cortelezzi A, Barranquero R, Marinelli C, Fernández San Juan R, Cepeda R. 2019. Environmental diagnosis of an urban basin from a social–ecological perspective. Sci Total Environ 678: 267–277. [Google Scholar]
- Cultid-Medina CA, Escobar F. 2016. Assessing the ecological response of Dung Beetles in an agricultural landscape using number of individuals and biomass in diversity measures. Environ Entomol 45: 310–319. [Google Scholar]
- Cummins KW, Klug MJ. 1979. Feeding ecology of stream invertebrates. Annu Rev Ecol Evol Syst 10: 147–172. [Google Scholar]
- Cummins KW, Merrit RW, Andrade PCN. 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud Neotrop Fauna Environ 40: 69–98. [CrossRef] [Google Scholar]
- Dahlgren R, Van Nieuwenhuyse E, Litton G. 2004. Transparency tube provides reliable water-quality measurements. Calif Agr 58: 149–153. [Google Scholar]
- Davis A. 1997. Climatic and biogeographical associations of southern African dung beetles (Coleoptera Scarabaeidae). Afr J Ecol 35: 10–38. [Google Scholar]
- de la Fuente EB, Suárez SA. 2008. Problemas ambientales asociados a la actividad humana: la agricultura. Ecol Austral 18: 239–252. [Google Scholar]
- de Melo SM, Takeda AM, Monkolski A. 2002. Seasonal dynamics of Callibaetis willineri (Ephemeroptera, Baetidae) associated with Eichhornia azurea (Pontedericeae) in Guaraná Lake of the Upper Paraná River, Brazil. Hydrobiologia 470: 57–62. [Google Scholar]
- Denegri MJ, Goldberg S, Parella M. 2014. Caracterización del clima de Luján. In: Carballo CT and Goldberg S (eds.), Comunidad e Información Ambiental del Riesgo: Las inundaciones y el río Luján. Argentina: Editorial Dunken, pp. 47. [Google Scholar]
- Domínguez E, Fernández HR, 2009. Macroinvertebrados bentónicos sudamericanos, Sistemática y biología. Tucumán, Argentina: Fundación Miguel Lillo p. 654. [Google Scholar]
- Dufrêne M, Legendre P. 1997. Species assemblages and indicators species: theneed for a flexible assymetrical approach. Ecol Monogr 67: 345–366. [Google Scholar]
- Ezcurra de Drago I, Marchese M, Montalto L. 2007. Benthic invertebrates. In: Iriondo MH, Paggi JC, and Parma MJ (eds.), The Middle Paraná River. Berlin: Springer, pp. 251–275. [Google Scholar]
- Feijoó CS, Giorgi A, García ME, Momo F. 1999. Temporal and spatial variability in streams of a pampean basin. Hydrobiologia 394: 41–52. [Google Scholar]
- Feld CK, Hering D. 2007. Community structure or function: effects of environmental stress on bentic macroinvertebrates at different spatial scales. Freshw Biol 52: 1380–1399. [Google Scholar]
- Ferreiro N, Feijoó C, Giorgi A, Leggieri L. 2011. Effects of macrophyte heterogeneity and food availability on structural parameters of the macroinvertebrate community in a Pampean stream. Hydrobiologia 664: 199–211. [CrossRef] [Google Scholar]
- Fidalgo F. 1983. Algunas características de los sedimentos superficiales en la cuenca del río Salado y en la Pampa ondulada. La Plata: Coloq. Int. Hidrologia de Grandes Fianuras, pp. 1–19. [Google Scholar]
- Firmiano KR, Ligeiro R, Macedo DR, Juen L, Hughes RM, Callisto M. 2017. Mayfly bioindicator thresholds for several anthropogenic disturbances in neotropical savanna streams. Ecol Indic 74: 276–284. [Google Scholar]
- Folke C, Jansson A, Larsson J, Costanza R. 1997. Ecosystem appropriation by cities. Ambio 26: 167–172. [Google Scholar]
- Giorgi A, Banchero M, Rivelli S, Clarensio O, Cuevas W. 1999. Algunas variables indicativas de la calidad del agua del río Luján en su tramo medio. Actas VII Jornadas Pampeanas de Ciencias Naturales, 155–162. [Google Scholar]
- Giorgi A, García ME, Feijoó C, Cuevas W, Vázquez Gómez A. 2000. Estudio comparativo de los principales arroyos afluentes del río Luján (Argentina). In: Pefaur JE (ed), Ecología Latinoamericana. Actas del III Congreso Latinoamericano de Ecología. Mérida: Editorial Universidad de Los Andes, pp. 99– 105. [Google Scholar]
- Grapentine LC, Rosenberg DM. 1992. Responses of the freshwater amphipod Hyalella azteca to environmental acidification. Can J Fish Aquat Sci 49: 52–64. [Google Scholar]
- Halstead JA, Kliman S, Berheide CW, Chaucer A, Cock-Esteb A. 2014. Urban stream syndrome in a small lightly developed watershed: a statistical analysis of water chemistry parameters land use patterns and natural sources. Environ Monit Assess 186: 3391–3414. [Google Scholar]
- Hwang SA, Hwang SJ, Park SR, Lee SW. 2016. Examining the relationships between watershed urban land use and stream water quality using linear and generalized additive models. Water 8: 155. [Google Scholar]
- Instituto Nacional del Agua. 2007. Diagnóstico del funcionamiento hidrológico hidráulico de la cuenca del río Luján, provincia de Buenos Aires. http://www.delriolujan.com.ar/estudioina.html (accessed May 15, 2019). [Google Scholar]
- Jayawickreme DH, Santoni CS, Kim JH, Jobbágy EG, Jackson RB. 2011. Changes in hydrology and salinity accompanying a century of agricultural conversion in Argentina. Ecol Appl 21: 2367–2379. [Google Scholar]
- Law al AT, Adeloju SB. 2013. Progress and recent advances in phosphate sensors: a review. Talanta 114: 191–203. [Google Scholar]
- Lombardo RJ, O'Farrell I, dos Santos Afonso M. 2010. Spatial and temporal ion dynamics on a complex hydrological system: the Lower Luján River (Buenos Aires, Argentina). Aquat Geochem 16: 293–309. [Google Scholar]
- Lozano F, Muzón J, Torres S. 2009. Description of the final instar larva of Homeoura lindneri (Ris, 1928) and redescription of the larva of H. chelifera (Selys, 1876) (Odonata: Coenagrionidae). Zootaxa 2231: 47–54. [Google Scholar]
- Manuel-Navarrete D, Gallopín GC, Blanco M, Díaz-Zorita M, Ferraro DO, Herzer H, Laterra P, Murmis MR, Podestá GP, Rabinovich J, Satorre EH, Torres F, Viglizzo EF. 2009. Multi-causal and integrated assessment of sustainability: the case of agriculturization in the Argentine Pampas. Environ Dev Sustain 11: 621–638. [Google Scholar]
- Martín SM, Díaz AC. 2012. Population structure of Uncancylus concentricus (d'Orbigny, 1835) (Ancylidae, Pulmonata, Basommatophora) in the Multiple Use Reserve Martín García Island, Upper Río de la Plata, Argentina. Braz J Biol 72: 65–70. [Google Scholar]
- Marzluff JM, Ewing K. 2001. Restoration of fragmented landscapes for the conservation of birds: a general framework and specific recommendation for urbanizing landscapes. Restor Ecol 9: 280–292. [Google Scholar]
- McCune B, Mefford MJ. 1999. Multivariate Analysis of Ecological Data, Version 4.25. Gleneden Beach, OR: MjM Software. [Google Scholar]
- McGeoch MA, Chown SL. 1998. Scaling up the value of bioindicators. Trends Ecol Evol 13: 46–47. [Google Scholar]
- McKinney ML. 2002. Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 42: 883–890. [Google Scholar]
- Medan D, Torretta JP, Hodara K, de la Fuente EB, Montaldo NH. 2011. Effects of agriculture expansion and intensification on the vertebrate and invertebrate diversity in the Pampas of Argentina. Biodivers Conserv 20: 3077–3100. [Google Scholar]
- Medina AI, Paggi AC. 2004. Composición y abundancia de Chironomidae (Diptera) en un río serrano de zona semiárida (San Luis, Argentina). Rev Soc Entomol Argent 63: 107–118. [Google Scholar]
- Medina AI, Scheibler EE, Paggi AC. 2008. Distribución de Chironomidae en dos sistemas fluviales ritrónicos (andino-serrano) de Argentina. Rev Soc Entomol Argent 67: 69–79. [Google Scholar]
- Meyer JL, Paul MJ, Taulbee WK. 2005. Stream ecosystem function in urbanizing landscapes. J North Am Benth Soc 24: 602–612. [Google Scholar]
- Milly PCD, Dunne KA, Vecchia AV. 2005. Global pattern of trends in streamflow and water availability in a changing climate. Nature 438: 347–350. [CrossRef] [PubMed] [Google Scholar]
- Miserendino ML. 2001. Macroinvertebrate assemblages in Andean Patagonian rivers and streams: environmental relationships. Hydrobiologia 444: 147–158. [Google Scholar]
- Miserendino ML, Brand C, Di Prinzio CY. 2008. Assessing urban impacts on water quality, benthic communities and fish in streams of the Andes Mountains, Patagonia (Argentina). Water Air Soil Pollut 194: 91–110. [Google Scholar]
- Miserendino ML, Brand C. 2009. Environmental effects of urbanization on streams and rivers in Patagonia (Argentina): the use of macroinvertebrates in monitoring. In: Justin Daniels A (ed.), Advances in Environmental Research. New York: NOVA pp. 183–220. [Google Scholar]
- Molineri C, Tejerina EG, Torrejón SE, Pero EJ, Hankel GE. 2020. Indicative value of different taxonomic levels of Chironomidae for assessing the water quality. Ecol Indic 108: 105703. [Google Scholar]
- Monk WA, Compson ZG, Choung CB, Korbel KL, Rideout NK, Baird DJ. 2019. Urbanisation of floodplain ecosystems: weight-of-evidence and network metaanalysis elucidate multiple stressor pathways. Sci Total Environ 684: 741–752. [Google Scholar]
- Moore AA, Palmer MA. 2005. Invertebrate biodiversity in agricultural and urban headwater streams: implications for conservation and management. Ecol Appl 15: 1169–1177. [Google Scholar]
- Moya C, Valdovino C, Moraga A, Romero F, Debels P, Oyanedel A. 2009. Patrones de distribución espacial de ensambles de macroinvertebrados bentónicos de un sistema fluvial Andino Patagónico. Rev Chil Hist Nat 82: 425–442. [Google Scholar]
- Muralidharan M, Selvakumar C, Sundar S, Raja M. 2010. Macroinvertebrates as potential indicators of environmental quality. Int J Biol Technol 1: 23–28. [Google Scholar]
- Naiman RJ, Décamps H. 1997. The ecology of interfaces: riparian zones. Annu Rev Ecol Syst 28: 621–658. [Google Scholar]
- Nieto C, Ovando XMC, Loyola R, Izquierdo A, Romero F, Molineri C, Rodríguez J, Rueda Martín P, Fernández H, Manzo V, Miranda MJ. 2017. The role of macroinvertebrates for conservation of freshwater systems. Ecol Evol 7: 5502–5513. [Google Scholar]
- Ocón C, Rodrígues Capítulo A. 2004. Presence and abundance of Ephemeroptera and other sensitive macroinvertebrates in relation with habitat conditions in pampean streams (Buenos Aires, Argentina). Arch Hydrobiol 159: 473–487. [Google Scholar]
- O'Farrell I, Lombardo R, De Tezanos Pinto P, López C. 2002. The assessment of water quality in the Lower Luján River (Buenos Aires, Argentina): phytoplankton and algal bioassays. Environ Pollut 120: 207–218. [Google Scholar]
- Ormerod SJ, Dobson M, Hildrew AG, Townsend DCR. 2010. Multiple stressors in freshwater ecosystems. Freshwater Biol 55: 1–4. [Google Scholar]
- Paggi AC, Ocón C, Tangorra M, Rodrigues Capítulo A. 2006. Response of the zoobenthos community along the dispersion plume of a highly polluted stream in the receiving waters of a large river (Rio de la Plata, Argentina). Hydrobiologia 568: 1–14. [Google Scholar]
- Palomeque R. 2007. Efectos del uso sobre algunas propiedades físicas y químicas del suelo y su utilización como indicadores de calidad. Trabajo Final de Aplicación. Luján: Universidad Nacional de Luján. [Google Scholar]
- Paul MJ, Meyer JL. 2001. Streams in the urban landscape. Annu Rev Ecol Syst 32: 333–365. [Google Scholar]
- Pizarro H, Alemanni ME. 2005. Variables físico-químicas del agua y su influencia en la biomasa del perifiton en un tramo inferior del Río Luján (Provincia de Buenos Aires). Ecol Austral 15: 73–88. [Google Scholar]
- Prat N. 1997. La problemática de la conservación de los ríos españoles como ecosistemas. Ecosistemas 20: 42–47. [Google Scholar]
- Prat N, Rieradevall M, 2014. Guía para el reconocimiento de las larvas de Chironomidae (DIPTERA) de los ríos mediterráneos, Grup de recerca F.E.M. (Freshwater Ecology and Management), Universidad de Barcelona, p. 29. [Google Scholar]
- Quantum GIS Geographic Information System. 2017. Open source geospatial foundation Project. http://qgis.osgeo.org. [Google Scholar]
- R Development Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Rocha L, Hegoburu C, Torremorel A, Feijoó C, Navarro E, Fernández H. 2020. Use of ecosystem health indicators for assessing anthropogenic impacts on freshwaters in Argentina: a review. Environ Monit Assess 192: 611. [Google Scholar]
- Rodrigues Capitulo A, Gómez N, Giorgi A, Feijoó C. 2010. Global changes in pampean lowland streams (Argentina): implications for biodiversity and functioning. Hydrobiologia 657: 53–70. [Google Scholar]
- Rodrigues Capítulo A, Armendáriz L, Siri A, Altieri P, Ocon C, Cortese B, Rodríguez Catanzaro L, Zanotto Arpellino JP, Rodríguez M, Donato M. 2020. Caracterización estructural y funcional de los macroinvertebrados en los bañados de desborde fluvial del área pampeana. Biol Acuática 35: 015–015. [Google Scholar]
- Rosenberg DM, Resh VH. 1993. Freshwater biomonitoring and benthic macroinvertebrates. London: Chapman and Hall, p. 488. [Google Scholar]
- Rosso JJ, Fernández Cirelli AF. 2013. Effects of land use on environmental conditions and macrophytes in prairie lotic ecosystems. Limnologica 43: 18–26. [Google Scholar]
- Ruiz-Picos RA, Sedeño-Díaz JE, López-López E, 2016. Ensambles de macroinvertebrados acuáticos relacionados con diversos usos del suelo en los ríos Apatlaco y Chalma-Tembembe (cuenca del Río Balsas), México. Hidrobiológica 26: 443–458. [Google Scholar]
- Rumi A, Gutiérrez Gregoric DE, Núñez V, Darrigran GA. 2008. Malacología Latinoamericana. Moluscos de agua dulce de Argentina. Rev Biol Trop 56: 77–111. [Google Scholar]
- Sabater S, Butturini A, Clement JC, Burt T, Dowrick D, Hefting M, Matre V, Pinay G, Postolache C, Rzepecki M, Sabater F, 2003. Nitrogen removal by riparian buffers along a European climatic gradient: patterns and factors of variation. Ecosystems 6: 20–30. [Google Scholar]
- Sala JM, Gonzalez N, Kruse E, 1983. Generalización hidrológica de la provincia de Buenos Aires. Coloquio Internacional sobre Hidrología de Grandes Llanuras 976–1009. [Google Scholar]
- Sánchez Caro A, Giorgi A, Doyle S, Piccinini M. 2012. La calidad del agua del Río Luján (Buenos Aires) y el potencial aporte del biofilm para su evaluación. Biol Acuática 27: 191–208. [Google Scholar]
- Sánchez-Pérez JM, Trémolières M, 2003. Change in groundwater chemistry as a consequence of suppression of floods: the case of the Rhine floodplain. J Hydrol 270: 89–104. [Google Scholar]
- Scheibler EE, Ciocco NF, 2011. Distribution of macroinvertebrate assemblages along a saline wetland in harsh environmental conditions from Central-West Argentina. Limnologica 41: 37–47. [Google Scholar]
- Serra SR, Graça MA, Dolédec S, Feio MJ, 2017. Chironomidae traits and life history strategies as indicators of anthropogenic disturbance. Environ Monit Assess 189: 326. [Google Scholar]
- Solis M, Arias M, Fanelli S, Bonetto C, Mugni H, 2019. Agrochemicals' effects on functional feeding groups of macroinvertebrates in Pampas streams. Ecol Indic 101: 373–379. [Google Scholar]
- Sponseller RA, Benfield EF, Valett HM, 2001. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshwater Biol 46: 1409–1424. [Google Scholar]
- Strickland JD, Parsons TR. 1968. A Practical Handbook of Seawater Analysis. Ottawa, ON: Fisheries Research Board of Canada, p. 310. [Google Scholar]
- Tagliaferro M, Giorgi A, Torremorell A, Albariño R. 2020. Urbanisation reduces litter breakdown rates and affects benthic invertebrate structure in Pampean streams. Int Rev. Hydrobiol. 105: 33–43. [Google Scholar]
- Taylor SL, Roberts SC, Walsh CJ, Hatt BE. 2004. Catchment urbanisation and increased benthic algal biomass in streams: linking mechanisms to management. Freshw Biol 49: 835–851. [Google Scholar]
- Tickner D, Armitage PD, Bickerton MA, Hall KA. 2000. Assessing stream quality using information on mesohabitat distribution and character. Aquat Conserv: Mar Freshw Ecosyst 10: 170–196. [Google Scholar]
- Tietze E. 2011. Distribución de Heleobia parchappii en ambientes dulceacuícolas de la Región Pampeana (Argentina). In: Cazzaniga NJ (ed.), El género Heleobia en América del Sur. Amici Molluscarum special number. Sociedad Malacológica de Chile (SMACH), pp. 73–75. [Google Scholar]
- Tietze E, Cristini PA, Grondona SI. 2019. Mollusk communities differ between microenvironments within a shallow lake in the Pampean Region, Argentina. Ecol Austral 29: 1–11. [Google Scholar]
- Townsend CR, Doledec S, Norris R, Peacock K, Arbuckle C. 2003. The influence of scale and geography on relationships between stream community composition and landscape variables: description and prediction. Freshw Biol 48: 768–785. [Google Scholar]
- van Rensburg BJ, McGeoch MA, Chown SL, van Jaarsveld AS. 1999. Conservation of heterogeneity among dung beetles in the Maputaland Centre of Endemism, South Africa. Biol Conserv 88: 145–153. [Google Scholar]
- Velázquez GA. 2013. La Calidad Ambiental en la Argentina: análisis regional y departamental. Tandil: Universidad Nacional del Centro de la Provincia de Buenos Aires. [Google Scholar]
- Vidaurre M, Pacheco LF, Roldán AI. 2006. Composition and abundance of birds of Andean alder (Alnus acuminata) patches with past and present harvest in Bolivia. Biol Conserv 132: 12–21. [Google Scholar]
- Viglizzo E, Jobbágy EG. 2010. Expansión de la frontera agropecuaria en Argentina y su impacto ecológico-ambiental. Buenos Aires: Ediciones INTA, p. 106. [Google Scholar]
- Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Reidy Liermann C, Davies PM. 2010. Global threats to human water security and river biodiversity. Nature 467: 555–561. [CrossRef] [PubMed] [Google Scholar]
- Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP. 2005. The urban stream syndrome: current knowledge and the search for a cure. J North Am Benth Soc 24: 706–723. [Google Scholar]
- Wantzen KM, Marchese MR, Marques MI, Battirola LD. 2016. Chapter 14: Invertebrates in Neotropical Floodplains. In: Batzer D and Boix D (eds.), Invertebrates in Freshwater Wetlands. Cham: Springer, pp. 493– 524. [Google Scholar]
- Webb JM, Suter PJ. 2011. Identification of Larvae of Australian Baetidae. Mus Vic Sci Rep 15: 1–24. [Google Scholar]
- Wetzel RG. 2001. Limnology: Lake and River Ecosystems. San Diego: Academic Press/Elsevier, p. 1006. [Google Scholar]
- Woodward G, Perkins DM, Brown LE. 2010. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos Trans R Soc B 365: 2093–2106. [Google Scholar]
- Xenopoulos MA, Lodge DM, Alcamo J, Märker M, Schulze K, Van Vuuren DP. 2005. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Global Change Biol 11: 1557–1564. [Google Scholar]
Cite this article as: Fañani AB, Cibils-Martina L, Casset MA, Banegas BP, Poretti TI, Rocha L. 2021. Specific indicator invertebrates of urbanized habitats in tributary streams of the Luján River basin (Buenos Aires, Argentina). Ann. Limnol. - Int. J. Lim. 57: 12
All Tables
Locations and environmental features of the study sites. The different land uses were determined with Quantum GIS 3.4.9.
Indicator values (IndVals) for invertebrate taxa of the sites with different condition of urbanization. Monte Carlo test was used to assess the significance of each taxa as an indicator for urbanization. Only taxa with significant indicator values (p < 0.05) are listed.
Mean values of physical and chemical parameters in each study site in four periods. In parentheses are the standard deviation values.
Mean abundance for four periods (standard deviation between brackets) and Functional Feeding Group (FFG) of invertebrate taxa at each site. References: GC (gatherers collectors), FC (filterers collectors), SH (shredders), SC (scrapers) and PR (predators).
All Figures
 |
Fig. 1 Location of the nine study sites in the upper, middle, and lower basin of the Luján River (Buenos Aires province). |
| In the text | |
 |
Fig. 2 Ordination plot according to the Principal Component Analysis of (a) environmental variables and (b) study sites. Variables: Nitrates (mg L−1), Phosphates (mg L−1), Turbidity (NTU), pH, Water Temperature (°C), DO (mg L−1), COD (mg O2.L−1), BOD5 (mg O2.L−1), SPOM (g L−1), SPIM (g L−1), Conductivity (μS cm−1), Urbanization (%). Sites: red crosses for high urbanization sites, blue circle for moderate urbanization sites and green triangles for low urbanization sites. |
| In the text | |
 |
Fig. 3 Percentages of invertebrate functional feeding groups (FFG) sampled from the study sites. References: SH: Shredder; FC: Collector-Filterer; SC: Scraper; GC: Gatherer-Collector; PR: Predator. |
| In the text | |
 |
Fig. 4 Rank-abundance curve of the three urbanization conditions. |
| In the text | |
 |
Fig. 5 Diagram of Non-metric multidimensional scaling (NMDS) of the invertebrate communities in three urbanization conditions. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


