| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 56, 2020
|
|
|---|---|---|
| Article Number | 9 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/limn/2020007 | |
| Published online | 30 April 2020 | |
Research Article
Land use change causes environmental homogeneity and low beta-diversity in Heteroptera of streams
1
Programa de Pós-Graduação em Biodiversidade e Conservação-PPGBC, Laboratório de Ictiologia de Altamira-LIA, Universidade Federal do Pará − UFPA, campus de Altamira, Pará, Brazil
2
Programa de Pós-Graduação em Zoologia − UFPA/MPEG, Laboratório de Ecologia e Conservação − LABECO, Instituto de Ciências Biológicas, Universidade Federal do Pará, Rua Augusto Corrêa, N° 1, Bairro Guamá, CEP: 66075-110, Belém, Pará, Brazil
3
Programa de Pós-Graduação em Biodiversidade e Conservação-PPGBC, Laboratório de Ictiologia de Altamira-LIA, Universidade Federal do Pará − UFPA, campus de Altamira, Pará, Brazil
4
Programa de Pós-Graduação em Ecologia e Conservação − PPGECO, Universidade do Estado de Mato Grosso − UNEMAT, campus de Nova Xavantina, Mato Grosso, Brazil
* Corresponding author: brasil_biologia@hotmail.com
Received:
9
January
2020
Accepted:
24
March
2020
Although species distribution pattern is a widely discussed topic, understanding the mechanisms that drive it in time and space is still one of the central goals of ecology. Moreover, it is of the most importance to discuss the maintenance of this biodiversity and the services it provides. Therefore, our aim is to test the following hypotheses: 1) Preserved environments have higher beta-diversity than environments with lower preservation values, since beta-diversity is determined by environmental variations between habitats; 2) Beta-diversity will be better than species richness to detect changes in community regarding environmental integrity gradients. This will occur because richness is not sensitive to changes in composition and this might mask results when sensitive species are lost and generalist species are introduced into the altered environments. In order to test these hypotheses, 20 points were sampled in five streams of the Brazilian Cerrado with different integrity conditions. Environmental change did not affect Heteroptera richness; however, it affected the beta-diversity of the group as a whole and of Nepomorpha, also negatively affecting both Gerromorpha beta-diversity and richness. Moreover, there was difference in variation of Gerromorpha composition in altered and degraded sites, but there was no effect on Nepomorpha. These results show that Gerromorpha is more sensitive to physical changes in streams caused by the loss of environmental integrity. Therefore, environmental changes with no regard to riparian vegetation boundaries causes shifts in stream conditions and changes aquatic communities, which places at risk the ecosystems services provided by these communities.
Key words: Gerromorpha / Nepomorpha / habitat integrity / limnological variables
© EDP Sciences, 2020
1 Introduction
Understanding the mechanisms that drive the distribution of organisms in space and time is still one of the central goals of ecology (Sutherland et al., 2013), which seeks to identify, describe, and explain the general patterns that are the major driving forces of community structure (Hubbell 2001; Leibold et al., 2004). The metapopulation theory formulated by Levins in 1969 is one of the theories designed to understand these community organization patterns. Levins proposed that populations of the same species might be structured in different ways, due to local extinction mechanisms and colonization by migrating individuals, derived from more stable populations (e.g., sites with more resources and genetic variability) (Hansky et al., 1996; Hansky and Simberloff 1997). Therefore, even sites with low amounts of resources or environmental conditions outside the range for species requirements might maintain biodiversity at a certain level due to source habitats, which would provide constant migration of species.
In order to quantify this spatial dynamics and the variation in species composition in communities, diversity can be divided into three scales, alpha (α) diversity, which is defined as the local diversity, or diversity within the habitat; gamma (γ) diversity, which is the total diversity of a region including several habitats; and beta (β) diversity (Buschini and Woiski 2008), which is the variation in species composition between sites or different habitats (Whittaker 1972). Alfa-diversity is traditionally called species richness and is one of the most frequently used measures to evaluate diversity patterns (Arrhenius 1921; MacArthur and Wilson 1967; Wright 1983), despite its limitation to equate all species. This problem is minimized in beta-diversity due to its potential to measure variation in species composition, which thus renders it a robust tool both to detect diversity patterns (Koleff et al., 2003; Harrison et al., 1992; Peixoto et al., 2017; Juen and De Marco 2011; Brasil et al., 2018) and to measure environmental impacts on biotic diversity (Quintana et al., 2017; Altermatt and Holyoak 2012; Ferreira et al., 2017). Beta-diversity is considered an important measure, providing a connection between alpha-diversity (local) and gamma-diversity (regional) (Vellend 2010).
The major hypotheses that explain differences in diversity use local, geographical, and/or historical environmental factors (Nekola and White 1999). Due to higher variation in environmental conditions, there is an increase in dissimilarity of species composition (beta-diversity) (Heino et al., 2015), which might in turn provide higher variation, in environmental conditions contemplating species with different environmental requirements. Thus, sites that are environmentally more heterogeneous would have higher beta-diversity values (Anderson et al., 2006). Considering spatial factors, sites that are farther from each other are expected to have higher dissimilarity in species composition (beta-diversity) (Legendre 1993). This is because the closer the areas are, the higher is the probability of migration within the landscape (Alahuhta and Heino 2013). However, under this spatial perspective, autoecological factors such as species dispersal ability and biogeographical factors, such as the presence of geo-graphical barriers, might limit dispersal and limit species diversity patterns across the landscape (Juen and De Marco 2012; Dambros et al., 2017; Brasil et al., 2018).
The constant conversion of natural landscape for anthropic purposes, e.g. agro-systems, urbanization, and/or industrialization (Brando et al., 2013; Gardner et al., 2013) is one of the great bottlenecks in maintaining biodiversity since it generally results in a simplification of environmental conditions, leading to habitat homogenization. Therefore, understanding how these environmental changes affect species, environmental conditions, and ecosystem services has become an increasingly pressing issue (Nessimian et al., 2008; Laurance et al., 2017). In this setting, evaluating variations in beta-diversity across environmental gradients with different environmental change levels is an important tool for biomonitoring (Ligeiro et al., 2013) as the local extinction of more specialist species is more frequent due to environmental impacts, because they tolerate few environmental changes. On the other hand, it is not rare for generalist species to enter these habitats, since they have more ability to disperse and tolerate higher variation in environmental conditions (Oliveira-Junior et al., 2015; 2019a). Therefore, this variation is not detected by alpha-diversity, as alpha-diversity is practically constant or might even increase. These characteristics indicate that evaluating changes in species composition across environmental gradients provide more robust results than traditional alpha-diversity measures (species richness) for the analyses of environmental impact on species distribution at a community level (Miguel et al., 2017)
In aquatic environments, aquatic insects are considered good models to evaluate how environmental changes affect environmental quality (Resh and Rosenberg, 1993). Among these organisms, Heteroptera stands out since they have a high dispersal ability and great potential as pioneer species in colonizing new water bodies (Bachmann 1998). Heteroptera are strongly dependent on riparian vegetation and are therefore good indicators of physical disturbances in streams (Dias-Silva et al., 2010; Cunha et al., 2015). Additionally, the removal of riparian vegetation leads to a disruption in physical characteristics of streams, increasing silting and destabilizing margins, affecting width, depth, and stream flow, which are very important metrics for the structuring of aquatic communities in streams.
In this scenario of environmental change, our aim was to evaluate the effect of environmental change on beta-diversity of aquatic and semi-aquatic Heteroptera in streams of the Cerrado, testing the following hypotheses: (1) preserved environments have higher beta-diversity than environments with lower preservation values; (2) beta-diversity shall be more sensitive than species richness in detecting changes in community related to environmental integrity gradients.
2 Material and methods
2.1 Study area
The sampling of aquatic and semi-aquatic Heteroptera was conducted in the Pindaíba River basin, a tributary of the right margin of the middle Rio das Mortes River, located in the eastern region of Mato Grosso, with an area of approximately 10,323 km2, in the Cerrado Biome. Its sources are located in the Acantilados Plateau, in the municipality of Barra do Garças.
1st and 4th-order stretches were sampled (Strahler, 1957) from 5 streams (Córrego da Mata (CRM), Córrego Caveira (CRCV), Taquaral (CRT), Cachoeirinha (CRC), and Papagaio (CRP)), totaling 20 sampling points (Fig. 1). The samples were realized in the month of September 2009, end of the dry season and beginning of the rainy season, so that samples from intermittent streams due to drought were not collected and the rainy season was avoided due to the filling of streams and the carrying of Gerromorpha by the water. The focus on a single seasonal period also reduces sampling “noise” in the analyses and results (Heino 2014). These streams have relatively similar features regarding the region of their sources. However, they differ in the conservation state of other stretches due to extensive cattle breeding and agricultural activities in the areas of Cachoeirinha and Caveira streams, whereas the riparian forests in Mata, Taquaral, and Papagaio streams were preserved.
Climate in the region is Aw type according to Koppen's classification, with mean annual temperature ranging from ±20 to ±26 °C and mean rainfall ranging from 1500 to 1600 mm, with two well-defined seasons; winter, less hot and dry (May to September) and a rainy summer (November to March).
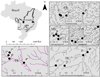 |
Fig. 1 Location of the 1st to 4th-order streams where the samplings of aquatic and semi-aquatic Heteroptera were conducted in 2008. |
2.2 Heteroptera sampling and identification
Samplings were conducted in fixed 100-m transects, divided in segments with five meters each (100/20 = 5). With the help of a net with 18 cm diameter (32 mm mesh size), three samples were collected in each segment in the water column (Cabette et al., 2010). Heteroptera were screened in the field, conserved in alcohol (85%) and identified in the laboratory with the help of dichotomous keys (Nieser and Melo 1997; Ribeiro 2005). Witness material is deposited at the Zoobotanical Collection “James Alexander Ratter” of UNEMAT, Nova Xavantina Campus.
2.3 Environmental conditions of streams
Environmental integrity was measured using the Habitat Integrity Index (HII) proposed by Nessimian et al. (2008). This index is comprised of 12 items that evaluate the structure of water bodies regarding riparian vegetation status, land use pattern beyond this range, retention devices, and types of substrate, aquatic vegetation, and detritus. HII has been widely used quite effectively as predictor of environmental conditions of streams about distribution of aquatic communities (Brasil et al., 2017; Vieira et al., 2015), and more specifically, for Heteroptera (Dias-Silva et al., 2010; Cunha and Juen 2017; Giehl et al., 2019).
Each of the 12 items in the HII is scored from 1 (very altered) to 4 or 5 (very preserved). This is because some items have 4 and other items have 5 response alternatives. For each item, in each stream the score obtained by the researcher is divided by the total number of options (4 or 5). This gives a partial score (12 points per stream). The final index is given by the sum of all partial scores divided by 12 (total number of items). In order to separate groups derived from sites considered either preserved or altered, streams with HII ≤ 0.6 were considered “altered” and sites with HII higher than that limit value were considered “preserved” (Brasil et al., 2017). Previous studies have already shown that these categories are efficient for classifying lotic environments according to their conservation category (Pereira et al., 2012; Oliveira-Junior et al., 2015; 2019b). In addition, the classification was also validated with observation by researchers in the field, where streams classified as altered due to heavy agricultural mechanization, the gallery forest at margins of these streams are highly deforested, replaced by pastures or soy monoculture, cattle raising. Other processes, such as erosion and silting are also observed. Since stream margins are exposed, the river channel lacks retention mechanisms and consequently little deposited organic material. On the other hand, for places characterized as preserved the predominant characteristics were: more than 50 m in width of gallery forest, continuously with the adjacent forest, retention mechanisms strongly fixed, lack of banks, little or no accumulation of sediments, riverbed, with stones grouped together, mosses and algae patches, and leaf detritus and woody material without sediment (Pereira et al 2012).
The physical-chemical variables were measured in the field and at the time of collection. Air temperature and water temperature were obtained with a digital thermometer (Multidigital) (Incoterm®), pH, turbidity, electrical conductivity, oxygen dissolved (OD) were obtained with a portable multi-parameter probe (Horiba®). For the analysis of phosphate, nitrate, nitrite and ammoniacal nitrogen we used a portable spectrophotometer (Hach®), and for the total hardness, calcium and magnesium we used the titulometric method with EDTA at 0.0002 M. Width measurements were obtained with a measuring tape (Leica DISTO TM®), the depth measurement was made using a depth meter (Sonar Echotest II®) and for the current speed we used a current meter (Geopacks MJP®). Flow data were calculated using the formula: Q = A × V, which represents the relationship between the area (A) of the channel cross section (width x average depth) and the speed of the current (V), being expressed as Q = L × P × V, where Q = flow, L = average width, P = average depth and V = average speed (Cunha and Guerra, 1996). All the variables mentioned above were measured at three points in each stream and the average values were used in the analyses.
2.4 Data analysis
The relationship of Heteroptera with environmental integrity gradients is idiosyncratic considering its infraorders Gerromorpha and Nepomorpha. Conditions concerning the physical part of the stream are mostly related to Gerromorpha and chemical conditions are mostly related with Nepomorpha; therefore, we analyzed both communities as a whole and its infraorders (Gerromorpha and Nepomorpha) separately in all tests (Dias-Silva et al., 2010). Additionally, the group of taxa selected for all analyses in each stream was considered a sampling unit.
Species richness was obtained based on the non-parametric estimator, first-order Jackknife, using the EstimateS Win 7.5 software (Colwell 2000). Beta-diversity was calculated using Sorensen's index modified by Chao et al., (2005). This index was chosen because it considers species abundance along with an estimate of species that have not been sampled (Chao et al., 2005). Moreover, it is considered relatively independent of species richness and more accurate even with small samples (Soininen et al., 2007). The higher the Sorensen's value, the higher the difference in species composition between two given communities. In order to find a beta-diversity value for each community, a mean value of comparisons (Sorensen's index) is calculated for the focal community with all the others in the background (Dambros et al., 2017; Brasil et al., 2018).
We performed simple linear regressions to test the hypothesis that there is a positive re-lationship between beta-diversity and environmental integrity, as well as the hypothesis that beta-diversity will be more effective than species richness in detecting changes in community regarding this environmental integrity gradient. Complementarily, we tested if there were differences between communities of preserved and degraded sites using a permutational analysis of dispersion homogeneity PERMANOVA (Permutational Multivariate Analyses of Variance) among the sampled sites using the “adonis” function (R vegan package) and PERMDISP (Permutational Analysis of Multivariate Dispersions) with the “betadisper” function (R vegan package) (Anderson et al., 2006). This test was performed based on the mean distances between points in a group of samples and the centroid of their multivariate space.
3 Results
3.1 Environmental conditions
Environmental integrity of streams varied between HII 0.52 and 0.96 (Tab. 2). Considering the integrity threshold value of HII = 0.6, eight streams were considered altered and twelve streams were considered preserved (Fig. 2a). Checking the environmental water conditions, we observed that streams considered to be preserved were more heterogeneous than the altered ones (Fig. 2b). The ordination of environmental conditions explained 57% of environmental variation in the first two axes and separated preserved streams from altered streams in the first axis (Fig. 2b). The most important environmental variables for this separation were pH, conductivity, hardness, Ca, and Mg, negatively related to the first axis, water and air temperature positively related to the second axis (Fig. 2c). Of the variables mentioned above, the highest differences occurred in water temperature, air temperature, both with higher values in degraded streams, and pH with the highest values in preserved streams (Fig. 2c-f).
Tests of difference (PERMANOVA) and homogeneity (PERMIDISP) in species composition of Heteroptera communities in preserved and altered streams in the Brazilian Cerrado.
Mean values of environmental variables in Mata Stream (MS), Cachoeirinha Stream (CS), Taquaral Stream (TS), Papagaio Stream (PS) and Caveira Stream (CVS). pH, conductivity eletrical (Eletrical. C.), turbidity (T.), Water Temperature (W.T.), Air Temperature (Air.T.), oxygen dissolved (O.D.), phosphate (P), hardness total (H), calcium (C), magnesium (Mg), nitrate (N), nitrite (Ni), Mean Width (M.W.), Mean depth (M. D.), flow (F) and HII for each streams and order sampled this study of Bioma Cerrado, Brazil.
 |
Fig. 2 Environmental variations in streams. Variations in HII values of preserved and altered streams (a), environmental heterogeneity in preserved and altered streams (b), ordination of environmental variables (c), variation in air temperature (d), water temperature (e) of preserved and altered streams, and variation in water pH of preserved and altered streams (f). |
3.2 Description of communities
A total of 2817 individuals were collected, distributed in 10 families, 32 genera, 13 species and 56 morphospecies. Most of the organisms were specimens of Gerromorpha, with 2294 individuals (81%), four families, 17 genera, and 31 morphospecies, and Nepomorpha, with 523 specimens (19%), six families, 13 genera, and 36 morphospecies/species (Tab. 3).
Species list and abundance of the individuals from Heteroptera in Cerrado Stream.
3.3 Environmental integrity and Heteroptera communities
Heteroptera richness (considering both infraorders together) was not affected by the environmental integrity gradient (r 2 = 0.139; p = 0.106) (Fig. 3d), and the same result was found for the infraorder Nepomorpha (r 2 = 0.00; p = 0.928) (Fig. 3f). However, Gerromorpha species richness had a significant positive relationship with environmental integrity gradient (r 2 = 0.227; p = 0.034) (Fig. 3b). Considering beta-diversity as a response variable, beta-diversity of both Heteroptera and Gerromorpha infraorder had a significant and positive relationship with the environmental integrity gradient (r 2 = 0.34; p = 0.007, and r 2 = 0.570; p < 0.001, respectively) (Fig. 3a and c). However, Nepomorpha beta-diversity had no relationship with the environmental integrity gradient (r 2 = 0.066; p = 0.274) (Fig. 3f).
Once streams were classified as either preserved (HII ≤ 0.6) or altered (HII > 0.6), there was no difference in species composition both of Heteroptera (Gerromorpha and Nepomorpha combined) and of Gerromorpha (PERMANOVA: pseudo-F = 2.136, R 2 = 0.106, p = 0.107 and pseudo-F = 2.100, R 2= 0.104, p = 0.098, respectively) between preserved and altered streams. However, there was difference in Nepomorpha species composition (PERMANOVA: pseudo-F = 3.225, R 2 = 0.151, p = 0.001) (Tab. 1). When we checked whether there was difference in the multivariate homogeneity of species composition in preserved and altered streams, both Heteroptera (Gerromorpha and Nepomorpha combined) and Gerromorpha had the most homogeneous communities at altered sites (PERMDISP: F = 5.875, p = 0.029 e F = 4.928, p = 0.039, respectively) (Tab. 1). The results of the multivariate homogeneity analysis are complementary evidence of the relative relationship between beta-diversity and HII (Fig. 3a and b), and both emphasize on community homogenization in streams that are environmentally more altered.
 |
Fig. 3 Effect of environmental integrity gradient measured by the Habitat Integrity Index (HII) on Gerromorpha beta-diversity (A) and Gerromorpha estimated species richness (B), Heteroptera beta-diversity (C) and estimated species richness (D) considering both infraorders together, and Nepomorpha beta-diversity (E) and Nepomorpha estimated species richness (F). |
4 Discussion
Overall, Heteroptera communities underwent changes due to loss of environmental integrity of streams. Beta-diversity was more sensitive to environmental integrity gradient than species richness. This positive relationship of beta-diversity with environmental integrity shows that pristine sites have a more dissimilar species composition than the background analyzed (all sites). This occurs because there are more rare species and/or species more sensitive to environmental changes at more preserved sites. On the other hand, community is more homogeneous at more altered sites, with predominant occurrence of more generalist species (Brasil et al., 2017). This causes species composition of the most altered site in the integrity gradient to be more similar to the background and this is reflected in its low beta-diversity values.
Sites that are environmentally more heterogeneous are expected to have more species, and this occurs because environmental heterogeneity provides more niche partitioning, which allows for a higher number of species with different environmental requirements to live sparingly, occupying complementary spaces in the environment (Tilman 1982; Cunha and Juen 2017). The lack of relationship of species richness with environmental integrity might occur when species change across the gradient of environmental conditions, especially with local extinction of more sensitive species and the intrusion of more generalist species (Dias-Silva et al., 2010; Cunha et al., 2015). This change cannot always be detected by species richness measures, as they are not sensitive to changes in species composition. However, changes can be detected by beta-diversity values (Carvalho and Gomes 2012), as we observed in the present study.
The positive effect of the Habitat Integrity Index on Heteroptera and Gerromorpha beta-diversity indicates that sites with higher integrity values harbor a higher diversity of these organisms and that these groups are related to habitat structure. Different impact intensities affect beta-diversity, and this can be caused by different mechanisms (Gutiérrez-Cánovas et al., 2013). One of the many factors associated with diversity studies is environmental heterogeneity (Death and Winterbourn 1995; Vieira et al., 2015; Alahuhta et al., 2017). In heterogeneous environments, species with different tolerance ranges tend to have differential spatial distribution patterns (Mykrä et al., 2007). Gerromorpha is a group that depends on the physical structure of streams, such as shading, channel retention material forming pools, or sites such as rapids. Therefore, when this structure is changed, this group responds more rapidly to these changes (Dias-Silva et al., 2010). So far, Nepomorpha has not shown to be sensitive to the physical changes that occurred in the studies we have evaluated, showing to be more sensitive to limnological variables. However, this response pattern cannot always be detected (Dias-Silva et al., 2010; Cunha et al., 2015), which leads us to believe that this group has a wide range of environmental plasticity.
For environmental monitoring, it is necessary to take some precautions when interpreting beta-diversity (Heino et al., 2015). Beta-diversity occurs due to turnover (exchange in species composition among sampling units) or nestedness (species richness gradient) (Baselga, 2010). Therefore, it is common to increase beta-diversity at large spatial scales due to species turnover related to biogeography (Alves-Martins et al., 2019; Brasil et al., 2018). This is not related to gradients of alteration of anthropic origin. On the other hand, in smaller spatial scales, beta-diversity reflects the nestedness nesting caused by loss of species sensitive to environmental changes (Heino et al., 2015). This creates a gradient of species richness. Therefore, the concomitant relationship between environmental integrity and species richness and beta-diversity in Gerromorpha and Heteroptera in our work demonstrates that there is a loss of species that causes the change in beta-diversity.
We have evidence that beta-diversity is more related to stream physical structure than species richness, and this is due to changes in species composition across the environmental gradient. Moreover, corroborating previous studies (Dias-Silva et al., 2010; Vieira et al., 2015), we emphasize that Gerromorpha are more sensitive to changes in environmental gradients and can therefore be used for these purposes. Overall, we indicated that the most altered sites located in pasture and/or agricultural areas are environmentally more homogeneous and have the lowest beta-diversity values. These results reinforce that changes in soil use without respecting riparian vegetation boundaries cause changes in environmental conditions, biodiversity, and might be changing the ecosystem services related to these communities.
Acknowledgements
We are grateful to Laboratório de Insetos Aquáticos de Nova Xavantina, FAPE-MAT, Proc. n° 098/2004 and 0907/2006. LJ received continuous research support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) productivity grants (Process 304710/2019-9). LSB thanks CAPES for the scholarship of the Programa Nacional de Pós-Doutorado (PNPD) linked to the Programa de Pós-Graduação em Zoologia da Universidade Federal do Pará (UFPA).
References
- Alahuhta J, Heino J. 2013. Spatial extent, regional specificity and metacommunity struc-turing in lake macrophytes. J Biogeogr 40: 1572–1582. [Google Scholar]
- Alahuhta J, Kosten S, Akasaka M, et al. 2017. Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. J Biogeogr 44: 1758–1769. [Google Scholar]
- Altermatt F, Holyoak M. 2012. Spatial clustering of habitat structure effects patterns of community composition and diversity. Ecology 93: 1125–1133. [PubMed] [Google Scholar]
- Alves-Martins F, Calatayud J, Medina NG, De Marco P, Juen L, Hortal J. 2019. Drivers of regional and local diversity of Amazonian stream Odonata. Insect Conserv Divers 12: 251–261. [Google Scholar]
- Anderson MJ, Ellingsen KE, McArdle BH. 2006. Multivariate dispersion as a measure of beta diversity. Ecol Lett 9: 683–693. [Google Scholar]
- Arrhenius O. 1921. Species and Area. J. Ecol 9: 95–99. [Google Scholar]
- Bachmann AO. 1998. Heteroptera acuáticos. Biodiversidad de Artrópodos Argentinos. Una Perspectiva Biotaxonómica. Ediciones Sur, La Plata. [Google Scholar]
- Baselga A. 2010. Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19: 134–143. [Google Scholar]
- Brando PM, Coe MT, DeFries R, Azevedo AA. 2013. Ecology, economy and management of an agroindustrial frontier landscape in the southeast Amazon. Phil Trans R Soc B 368: 20120152. [CrossRef] [Google Scholar]
- Brasil LS, Vieira TB, Oliveira-Junior JMB, Dias-Silva K, Juen L. 2017. Elements of metacommunity structure in Amazonian Zygoptera among streams under different spatial scales and environmental conditions. Ecol Evol 7: 3190–3200. [PubMed] [Google Scholar]
- Brasil LS, Oliveira-Júnior JM, Calvão LB, et al. 2018. Spatial, biogeographic and environmental predictors of diversity in A mazonian Zygoptera. Insect Conserv Divers 11: 174–184. [Google Scholar]
- Buschini MLT, Woiski TD. 2008. Alpha–beta diversity in trap-nesting wasps (Hymenoptera: Aculeata) in Southern Brazil. Acta Zool 89: 351–358. [Google Scholar]
- Cabette HSR, Giehl NFS, Dias-Silva K, Juen L, Batista JD. 2010. Distribuição de Nepomorpha e Gerromorpha (Insecta: Heteroptera) da Bacia do Rio Suiá-Miçú, MT: riqueza relacionada à qualidade de água e de hábitat, in Santos JE, Galbiati C, Moschini LE. (Orgs.). Gestão e Educação Ambiental: Água, Biodiversidade e Cultura. São Carlos, RiMa, pp. 113–137. [Google Scholar]
- Chao A, Chazdon RL, Colwell RK, Shen TJ. 2005. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8: 148–159. [Google Scholar]
- Carvalho JC, Cardoso P, Gomes P. 2012. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Global Ecology and Biogeography 21: 760–771. [CrossRef] [Google Scholar]
- Colwell RK. 2000. EstimateS version 6.0 b1: Statistical estimation of species richness and shared species from samples. Freeware published at http://viceroy.eeb.uconn.edu/EstimateS. [Google Scholar]
- Cunha SB, Guerra AJT. 1996. Degradação Ambiental. In: Cunha, SB Guerra, AJT. Geomorfologia e Meio Ambiente. Rio de Janeiro: Bertrand Brasil, pp. 337–379. [Google Scholar]
- Cunha EJ, Juen L. 2017. Impacts of oil palm plantations on changes in environmental heterogeneity and Heteroptera (Gerromorpha and Nepomorpha) diversity. J Insect Conserv 21: 111–119. [Google Scholar]
- Cunha EJ, de Assis Montag LF, Juen L. 2015. Oil palm crops effects on environmental integrity of Amazonian streams and Heteropteran (Hemiptera) species diversity. Ecol Indic 52: 422–429. [Google Scholar]
- Dambros CS, Morais JW, Azevedo RA, Gotelli NJ. 2017. Isolation by distance, not rivers, control the distribution of termite species in the Amazonian rain forest. Ecography 40: 1242–1250. [Google Scholar]
- Death RG, Winterbourn MJ. 1995. Diversity patterns in stream benthic invertebrate communities: the influence of habitat stability. Ecology 76: 1446–1460. [Google Scholar]
- Dias-Silva K, Cabette HSR, Juen L, De Marco P. 2010. The influence of habitat integrity and physical-chemical water variables on the structure of aquatic and semiaquatic Heteroptera. Zoologia 27: 918–930. [CrossRef] [Google Scholar]
- Ferreira WR, Hepp LU, Ligeiro R, et al. 2017. Partitioning taxonomic diversity of aquatic insect assemblages and func-tional feeding groups in neotropical savanna headwater streams. Ecol Indic 72: 365–373. [Google Scholar]
- Gardner TA, Ferreira J, Barlow J, et al. 2013. A social and ecological assessment of tropical land uses at multiple scales: the Sus-tainable Amazon Network. Phil Trans R Soc B 368: 20120166. [CrossRef] [Google Scholar]
- Giehl NFS, Brasil LS, Dias-Silva K, Nogueira D, Cabette HSR. 2019. Environmental Thresholds of Nepomorpha in Cerrado Streams, Brazilian Savannah. Neotrop Entomol 48: 186–196. [CrossRef] [PubMed] [Google Scholar]
- Gutiérrez-Cánovas C, Millán A, Velasco J, Vaughan IP, Ormerod SJ. 2013. Con-trasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Global Ecol Biogeogr 22: 796–805. [CrossRef] [Google Scholar]
- Hansky I, Simberloff D. 1997. The metapoplulation approach, its history, conceptual domain and application to conservation, in Hansky I, Simber-loff D. (eds.), Metapopulation Biology. Academic Press, San Diego, California, pp. 5–26. [CrossRef] [Google Scholar]
- Hansky I, Moilanen A, Gyllenberg M. 1996. Minimum viable metapopulation size. Am Nat 147: 527–541. [Google Scholar]
- Harrison S, Ross SJ, Lawton JH. 1992. Beta diversity on geographic gradients in Britain. J Animal Ecol 1: 151–158. [CrossRef] [Google Scholar]
- Heino J, Melo AS, Bini LM. 2015. Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw Biol 60: 223–235. [Google Scholar]
- Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton: Princeton University Press. [Google Scholar]
- Juen L, De Marco P. 2011. Odonate biodiversity in terra-firme forest streamlets in Central Amazonia: on the relative effects of neutral and niche drivers at small geographical extents. Insect Conserv Divers 4: 265–274. [Google Scholar]
- Juen L, De Marco P. 2012. Dragonfly endemism in the Brazilian Amazon: competing hypotheses for biogeographical patterns. Biodivers Conserv 21: 3507–3521. [Google Scholar]
- Koleff P, Gaston KJ, Lennon JJ. 2003. Measuring beta diversity for presence-absence data. J Animal Ecol 72: 367–382. [CrossRef] [Google Scholar]
- Laurance WF, Camargo JL, Fearnside PM, et al. 2017. An Amazonian rainforest and its fragments as a laboratory of global change. Biol Rev 93: 223–247. [CrossRef] [Google Scholar]
- Legendre P. 1993. Spatial autocorrelation: trouble or new paradigm? Ecology 74: 1659–1673. [Google Scholar]
- Leibold MA, Holyoak M, Mouquet N, et al. 2004. The metacommunity concept: a framework for multi − scale community ecology. Ecol Lett 7: 601–613. [Google Scholar]
- Ligeiro R, Hughes RM, Kaufmann PR, et al. 2013. Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecol Indic 25: 45–57. [Google Scholar]
- MacArthur RH, Wilson EO. 1967. The Theory of Island Biogeography. Princeton, NJ : Princeton University Press. [Google Scholar]
- Miguel TB, Oliveira-Junior JMB, Ligeiro R, Juen L. 2017. Odonata (Insecta) as a tool for the biomonitoring of environmental quality. Ecol Indic 81: 555–566. [Google Scholar]
- Mykrä H, Heino J, Muotka T. 2007, Scale-related patterns in the spatial and environ-mental components of stream macroinvertebrate assemblage variation. Global Ecol Biogeogr 16: 149–159. [CrossRef] [Google Scholar]
- Nekola JC, White PS. 1999. The distance decay of similarity in biogeography and ecology. J Biogeogr 26: 867–878. [Google Scholar]
- Nessimian JL, Venticinque EM, Zuanon J, et al. 2008. Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 614: 117. [Google Scholar]
- Nieser N, Melo AL. 1997. Os Heterópteros aquáticos de Minas Gerais. Guia introdutó-rio com chave de identificação para as espécies de Nepomorpha e Gerromorpha. Belo Horizonte, Univ. Fed. Minas Gerais. [Google Scholar]
- Oliveira-Júnior JMB, Juen L. 2019a. The Zygoptera/Anisoptera Ratio (Insecta: Odonata): a New Tool for Habitat Alterations Assessment in Amazonian Streams. Neotrop Entomol 48: 552–560. [CrossRef] [PubMed] [Google Scholar]
- Oliveira-Júnior JMB, Juen L. 2019b. Structuring of dragonfly communities (Insecta: Odonata) in eastern Amazon: effects of environmental and spatial factors in preserved and altered streams. Insects 10: 1–18. [Google Scholar]
- Oliveira-Junior JMB, Shimano Y, Gardner TA, Hughes RM, Marco Júnior P, Juen L. 2015. Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condi-tion of small streams in the eastern Amazon. Austral Ecol 40: 733–744. [Google Scholar]
- Pereira LR, Cabette HSR, Juen L. 2012. The use of aquatic insects of order Trichoptera as bioindicators of habitat integrity. Ann Limnol 48: 303–313. [Google Scholar]
- Peixoto FP, Villalobos F, Melo AS, et al. 2017. Geographical patterns of phylogenetic beta-diversity components in terrestrial mammals. Global Ecol Biogeogr 26: 573–583. [CrossRef] [Google Scholar]
- Quintana C, Girardello M, Barfod AS, Balslev H. 2017. Diversity patterns, envi-ronmental drivers and changes in vegetation composition in dry inter-Andean valleys. J Plant Ecol 10: 461–475. [Google Scholar]
- Resh VH, Rosenberg DM. 1993. Freshwater biomonitoring and benthic macroinver-tebrates. Amsterdam: Springer. [Google Scholar]
- Ribeiro JRI. 2005. Família Belostomatidae Leach, 1815 (Insecta: Hemiptera: Heterop-tera): chave e catálogo de identificação para as espécies ocorrentes no Estado do Rio de Janei-ro, Brasil. Arquivos do Museu Nacional, Rio de Janeiro 63: 247–262. [Google Scholar]
- Soininen J, Lennon JJ, Hillebrand H. 2007. A multivariate analysis of beta diversity across organisms and environments. Ecology 88: 2830–2838. [PubMed] [Google Scholar]
- Sutherland WJ, Freckleton RP, Godfray HCJ, et al. 2013. Identification of 100 fundamental ecological questions. J Ecol 101: 58–67. [Google Scholar]
- Tilman D. 1982. Resource Competition and Community Structure. Princeton University Press, Princeton, p. 296. [Google Scholar]
- Vellend M. 2010 Conceptual synthesis in community ecology. Q Rev Biol 85: 183–206. [CrossRef] [PubMed] [Google Scholar]
- Vieira TB, Dias-Silva K, Pacífico ES. 2015. Effects of riparian vegetation integrity on fish and heteroptera communities. Appl Ecol Environ Res 13: 53–65. [Google Scholar]
- Whittaker RH. 1972. Evolution and measurement of species diversity. Taxon 1: 213–251. [Google Scholar]
- Wright DH. 1983. Species-Energy Theory: An Extension of Species-Area Theory. Oikos 41: 496–506. [Google Scholar]
Cite this article as: Dias-Silva K, Brasil LS, Veloso GKO, Cabette HSR, Juen L. 2020. Land use change causes environmental homogeneity and low beta-diversity in Heteroptera of streams. Ann. Limnol. - Int. J. Lim. 56: 9
All Tables
Tests of difference (PERMANOVA) and homogeneity (PERMIDISP) in species composition of Heteroptera communities in preserved and altered streams in the Brazilian Cerrado.
Mean values of environmental variables in Mata Stream (MS), Cachoeirinha Stream (CS), Taquaral Stream (TS), Papagaio Stream (PS) and Caveira Stream (CVS). pH, conductivity eletrical (Eletrical. C.), turbidity (T.), Water Temperature (W.T.), Air Temperature (Air.T.), oxygen dissolved (O.D.), phosphate (P), hardness total (H), calcium (C), magnesium (Mg), nitrate (N), nitrite (Ni), Mean Width (M.W.), Mean depth (M. D.), flow (F) and HII for each streams and order sampled this study of Bioma Cerrado, Brazil.
Species list and abundance of the individuals from Heteroptera in Cerrado Stream.
All Figures
 |
Fig. 1 Location of the 1st to 4th-order streams where the samplings of aquatic and semi-aquatic Heteroptera were conducted in 2008. |
| In the text | |
 |
Fig. 2 Environmental variations in streams. Variations in HII values of preserved and altered streams (a), environmental heterogeneity in preserved and altered streams (b), ordination of environmental variables (c), variation in air temperature (d), water temperature (e) of preserved and altered streams, and variation in water pH of preserved and altered streams (f). |
| In the text | |
 |
Fig. 3 Effect of environmental integrity gradient measured by the Habitat Integrity Index (HII) on Gerromorpha beta-diversity (A) and Gerromorpha estimated species richness (B), Heteroptera beta-diversity (C) and estimated species richness (D) considering both infraorders together, and Nepomorpha beta-diversity (E) and Nepomorpha estimated species richness (F). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


