| Issue |
Int. J. Lim.
Volume 58, 2022
|
|
|---|---|---|
| Article Number | 7 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/limn/2022005 | |
| Published online | 28 June 2022 | |
Research Article
Longitudinal dynamics of Odonata assemblages in an anthropogenically impacted lotic system
1
Elektroprojekt d.d., Civil and Architectural Engineering Department, Water Resources, Nature and Environmental protection Section, Alexandera von Humboldta 4, Zagreb, Croatia
2
University of Zagreb, Faculty of Science, Department of Biology, Rooseveltov trg 6, Zagreb, Croatia
3
University of Zagreb, Faculty of Teacher Education, Trg Matice Hrvatske 12, Petrinja, Croatia
4
University of Zagreb, Faculty of Science, Department of Biology, Rooseveltov trg 6, Zagreb, Croatia
* Corresponding author: iva.vidakovic@elektroprojekt.hr
Received:
7
October
2021
Accepted:
14
March
2022
European lowland rivers are extensively impacted by hydromorphological pressures, and the relationship between individual benthic macroinvertebrate groups and these degradations are insufficiently investigated. Therefore, we studied distribution and ecological traits of Odonata inhabiting a lotic system in the Pannonian lowland ecoregion (ER 11) in Croatia affected by degraded water quality and hydromorphological stressors. The study encompassed multihabitat sampling of 20 longitudinally distributed sampling sites, selected for their representativeness of disturbances. Only five species were recorded with Platycnemis pennipes (Pallas, 1771) and Onychogomphus forcipatus (Linnaeus, 1758) dominating. We found woody debris samples contained a disproportionately higher number of Odonata, especially Zygoptera, compared to all other sampled microhabitats. The downstream longitudinal increase in Odonata abundance was not followed by an expected increase in species richness. Only five (oxygen saturation, pH, ammonium, water temperature and total nitrogen) of the sixteen tested water quality parameters were significant variables in determining the variation of Odonata assemblages. Calopteryx virgo (Linnaeus, 1758) and juvenile Gomphidae were associated with sites of somewhat higher ammonium and total nitrogen concentrations while all other taxa showed a negative association to ammonium. Odonata abundances were affected by modification of the river channel where significant decrease in abundance was observed with increased modification. Our results suggest that even species with a broad ecological tolerance are sensitive to hydromorphological pressures and represent an important background for further research and conservation practices of lotic European Odonata.
Key words: Dragonflies / damselflies / hydromorphology / water quality / Pannonian lowland ecoregion / microhabitats
© EDP Sciences, 2022
1 Introduction
Odonates (dragonflies and damselflies) can be found at a wide range of freshwater habitats, but very often, species are confided to predominantly lotic or lentic ones (Corbet, 1999; Askew, 2004). In Europe, of the 143 known species of Odonata, 44 species are classified as predominantly breeding in lotic habitats with southwest Europe holding the greatest diversity of lotic species (Kalkman et al., 2018; Boudot and Kalkman, 2015). Unfortunately, lotic systems are amongst the most degraded ecosystems on Earth (Giller and Malmqvist, 1998; Dudgeon, 2013), and European rivers, especially lowland ones, are no exception (Schinegger et al., 2012). River regulations not only cause alterations to instream physical habitats but also influence hydrological and physicochemical conditions within the river system altogether leading to considerable habitat loss (e.g. Ward and Stanford, 1979; Strayer 2006) and a dramatic decrease of freshwater biodiversity (Zwick, 1992; Céréghino et al., 2002; Poff and Zimmerman, 2010).
Odonata are a hemimetabolous merolimnic insect order spending their nymphal life in aquatic habitats and using terrestrial habitats as aerial adults (Kalkman et al., 2008). Sensitivity of nymphs and adults to changes in environmental conditions makes them excellent biological indicators in aquatic ecology research and conservation management (Moore, 1997; Clausnitzer, 2003; Suhling et al., 2006; Simaika and Samways, 2008; Harabiš and Dolný, 2012). They are a popular group in conservation biology, and with their umbrella role, they can serve in the protection of numerous taxa of flora and fauna (Rouquette and Thompson, 2005; Suh and Samways, 2005; Bried et al., 2007).
While longtime Odonata research in Northern and Central European freshwater ecosystems has resulted in relatively extensive knowledge of their ecology in various habitats (e.g. Chovanec et al., 2004; 2015; Harabiš and Dolný, 2010; Villalobos-Jiménez et al., 2016; Bowler et al., 2021) including knowledge on their response to climate change (Ott, 2010; Termaat et al., 2019), there are still large gaps in our knowledge of lotic Odonata ecology and distribution in Southeastern Europe, limiting species protection and management of important habitats. Therefore, the main goals of this study were to determine: (i) composition, abundance and longitudinal distribution of Odonata assemblages, (ii) their habitat and microhabitat preferences and (iii) influence of environmental variables and anthropogenic stressors along a lotic system in the Pannonian lowland ecoregion (ER11).
2 Materials and methods
2.1 Study area
The Bednja River, with a total length of 105 km, is located in northern Croatia and as a tributary of the Drava River it is part of the Danube River Basin. The entire catchment belongs to Pannonian lowland ecoregion (ER11). From its source at the foothills of Ravna Gora Mountain to its mouth into the Drava River, the Bednja River belongs to two river types: Mountain and foothill small stream (study sites 1–9) to Lowland mid-sized and large stream (study sites 10–20) (Fig. 1). The boundary for this typology change is marked by the altitude drop below 200 m a.s.l. The study sites were selected because they represent different levels of anthropogenic habitat disturbance, primarily hydromorphological alterations, but they also included areas of minimal human impact. Majority of the floodplain is used for intensive and extensive agriculture but areas along the river are also designated Natura 2000 sites. The river experiences a first maximum discharge in March or April and the second, heavier, in December, caused by heavy rainfall/snowmelt (Čanjevac, 2013). The minimum discharge periods are in August and February (Čanjevac, 2013). Köppen climate classification places the Bednja catchment under type Cfb, corresponding to regions with a temperate humid climate with warm summers where the average temperature of the coldest month does not drop below –3 °C and the average temperature of the warmest month does not exceed 22 °C (Šegota and Filipčić, 2003). The mean monthly air temperature in the study area during our study in 2015 was 11.6 °C (±7.6 °C), while the mean total yearly precipitation was 897 mm (±114.6 mm) (source: personal analysis from raw data received from Croatian Meteorological and Hydrological Service by request).
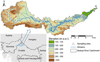 |
Fig. 1 Position of the Bednja River Catchment in Croatia and distribution of the 20 study sites along the Bednja River with respect to altitude. |
2.2 Sampling and laboratory methods
Odonata nymphs, together with other benthic macroinvertebrates, were collected in summer 2015 at 20 study sites distributed longitudinally along the course of the Bednja River (Fig. 1). Sampling site selection involved examination of physical plans to encompass areas upstream and downstream of wastewater outlets and a priori assessment of hydromorphological status. Samples were taken using the “multihabitat method” as presented in AQEM manual (AQEM consortium, 2002) with a standardized 500 μm mesh size, 25 × 25 cm frame hand net. At each study site, the distribution of microhabitats within a 100 m river reach was identified prior to sampling to avoid disturbance of the substrate and fauna. Within each sampling reach, 20 subsamples distributed according to share of microhabitat were taken, totalling 400 subsamples and covering two km of the river. Subsamples from each site were preserved in 96% ethanol. Sorting of benthic invertebrates was performed on entire samples, isolating all present organisms per sampled microhabitat under a stereomicroscope. Nymph individuals were identified to the lowest possible taxonomic level using Gerken and Sternberg (1999) combined with Askew (2004) and Brochard et al. (2012).
2.3 Environmental variables
Physicochemical water properties and nutrients were sampled parallel to benthic macroinvertebrate sampling at each study site parallel to macroinvertebrate sampling: water temperature (°C, using the oximeter WTW Oxi 330/SET), pH value (using the pH meter WTW pH 330), conductivity (µS/cm, using the conductometer WTW LF 330), oxygen regime: dissolved oxygen, oxygen saturation (%, calculated through dissolved oxygen and water temperature) and biological oxygen demand (BOD). Additionally, at each sampling site, one L of water was collected and analysed in the laboratory for the following parameters: total nitrogen, nitrites, nitrates, organic nitrogen, Kjeldahl nitrogen, ammonium, total phosphorous and orthophosphates. Water chemistry analyses were carried out according to standard methods (APHA, 1992). Water velocity (with P-670-M velocimeter) and water depth (with handheld meter) were measured above each subsample. Altitude and distance from the source for each study site were calculated in GIS tool ArcMap, version 10 (ESRI, 2010) (Tab. 1, Tab. S1).
Summary of environmental variables at the 20 study sites given as range of measured values.
2.4 Hydromorphological modification and naturalness of riparian zone
The degree of hydromorphological modification at each study site was assessed using the European Standard EN 15843:2010 (DIN, 2010) which assesses the “departure from naturalness as a result of human pressures on river hydromorphology”. It is based on the assessment of 16 individual hydromorphological features including scoring of the vegetation type and structure on riverbanks and adjacent land (See Supplementary Tab. S2). The assessment was performed on a 500 m long reach encompassing the 100 m sampled reach and extending upstream to account for drift. A single mean score for the assessed reach (study site) was derived by calculating the mean value for all 16 features assessed. The three separate scores for river zone were derived by calculating the mean score for features corresponding to river channel, bank/riparian zone and floodplain. For assessment of feature “Vegetation type/structure on banks and adjacent land” (Fig. 2), scoring is given for percentage of affected bank length on a scale of 1–5 as follows: 1 = 0% to 5% non-natural land cover in riparian zone, 2 > 5% to 15% non-natural land cover in riparian zone, 3 > 15% to 35% non-natural land cover in riparian zone, 4 > 35% to 75% non-natural land cover in riparian zone and 5 > 75% non-natural land cover in riparian zone. Hydromorphological modification scores are grouped into five classes representing different degrees of hydromorphological modification, from near natural to severely modified.
Odonata abundance given as number of individuals per meter squared. Only study sites and microhabitats where nymphs were recorded are given. Microhabitat abbreviations: MESO = mesolithal (hand-sized cobbles); MICRO = microlithal (coarse gravel); AKA = akal (fine to medium-sized gravel); PSA = psammal (sand); XYL = xylal (woody debris); ARG = argyllal (clay); TECHNO = technolithal (artificial blocks); MACROP = both submerged and emergent macrophytes. * - juvenile instars not identified to species level.
 |
Fig. 2 Hydromorphological modification at each study site according to norm EN 15843:2010. The mean score is derived from 16 individual features assessed. Grouped scores are given for modification of river channel, banks and riparian zone and floodplain. Individual score is given only for vegetation type / structure on banks and adjacent land. |
2.5 Data analysis
Spearman’s correlation coefficient (R) was used to determine significant correlations between altitude and distance from the source with total Odonata abundance and species richness. The same test was used to determine if water quality and hydromorphological status had a longitudinal gradient by testing the water quality variables and hydromorphological scores with distance from the source.
All pairwise distances of Odonata assemblages among different study sites were calculated with Bray-Curtis similarity index. All data were log (x + 1) transformed prior to analysis. A multidimensional scaling (MDS) plot was constructed to visually display the effects of different river reaches in shaping of Odonata assemblages and portray the results of hierarchical cluster analysis. Distances of Odonata assemblages among different microhabitats were also calculated with the Bray-Curtis similarity index and tested using hierarchical cluster analysis. The contribution of specific species to dissimilarity between assemblages of different microhabitats was tested using SIMPER analysis.
Canonical correspondence analysis (CCA) was used for identification and measurement of Odonata and environmental properties. This analysis was performed using data for all Odonata taxa, except species with a single occurrence (i.e. Orthetrum albistylum), with original scale abundance data (number of individuals per meter squared) and five significant water quality parameters (oxygen saturation, pH, ammonium, water temperature and total nitrogen concentration). In order to assess deviation from a randomly generated distribution, a Monte Carlo estimation (999 permutations) was applied to the procedure. Arrow lengths show the relative importance of the explanatory variables (water quality parameters), and their directions indicate positive or negative correlations.
Odonata abundance and species richness were correlated with hydromorphological modification scores in order to test the effect of hydromorphological degradation on Odonata assemblages using Spearman’s correlation coefficient. Species response curves (GAM – fit generalized additive model for each selected response variable) were constructed against the mean score for the degree of hydromorphological modification.
Spearman’s correlation coefficient was calculated using Statistica, 13.0 (TIBCO Software Inc., 2017). The Bray-curtis similarity index, Cluster and SIMPER analyses were performed using PRIMER 6 software package (Clarke and Gorley, 2006). The CCA analysis and species response curve were conducted in the CANOCO package version 5.0 (Ter Braak and Šmilauer, 2012).
3 Results
3.1 Environmental conditions on a longitudinal gradient
Physicochemical water properties and nutrients of the Bednja River were tested on a longitudinal scale, using distance from the source as a measure of longitudinal scaling. Water velocity (R = 0.243, p < 0.05), oxygen saturation (R = 0.331, p < 0.05), temperature (R = 0.492, p < 0.05), nitrites (R = 0.355; p < 0.05) and pH (R = 0.610, p < 0.01) were higher with increased distance from the source. Nutrients: total phosphorus (R = 0.561, p < 0.05), orthophosphates (R = 0.940; p < 0.001), nitrates (R = 0.888, p < 0.001) and total nitrogen concentrations (R = 0.754, p < 0.001) also showed positive correlations to distance from the source. Ammonium concentration (R = –0.373, p < 0.05) was the only parameter that significantly decreased with increased distance from the source. The values of measured water quality parameters (given in Tab. 1 and Supplementary Tab. S1.) show that the studied sites exhibit a water quality range from very good to moderate pollution loading.
3.2 Hydromorphological modification and naturalness of riparian zone
Four study sites (1, 4, 16 and 20) are reaches of the Bednja River not subject to recent management activities and have a class 1 mean score representing near natural hydromorphological conditions. Eight study sites (3, 8, 9, 12, 13, 14, 15 and 18) have a class 2 mean score representing slightly modified hydromorphological conditions. Five study sites (2, 5, 7, 10 and 19) are moderately modified (class 3) and three study sites (6, 11 and 17) are extensively modified (class 4). No study site received a class 5 (severely modified) hydromorphological status. Only one study site possesses near natural vegetation structure on banks and adjacent land (study site 4) while the remaining study sites are characterised by different levels of riparian zone degradation. Results for assessment of hydromorphological modification and naturalness of riparian vegetation at study sites are given in Figure 2 and Supplementary Table S2. Spearman’s correlation coefficient showed no significant correlations between hydromorphological modification scores and distance from source or altitude.
3.3 Odonata assemblages, species distribution and abundance
In total five different species were identified to species level from the 341 collected nymph individuals. Juvenile instars not possible to identify to species level belonged to the families Gomphidae, Libellulidae/Corduliidae, Coenagrionidae and suborder Zygoptera. The most numerous taxon was Coenagrionidae, while Platycnemis pennipes (Pallas, 1771) was the most numerous species. Onychogomphus forcipatus (Linnaeus, 1758) was the most widespread, recorded at 14 of the 20 study sites (Tab. 2). None of the identified species are listed under the Annexes of the EU Habitats Directive. Odonata assemblages were absent from only two sites: Study site 1, the most upstream study site near the source and study site 19. In general, Odonata abundances were higher with increased distance from the source (R = 0.297; p < 0.05) and consequently lower with increased altitude (R = –0.298; p < 0.05). Differences in species richness did not show statistical significance with either distance from the source or altitude. However, some grouping of similar Odonata assemblages from different study sites is visible among different river reaches of Bednja, and it is clearly not related to the position of the study sites along the river’s course (Fig. 3).
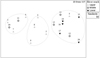 |
Fig. 3 Position of the study site Odonata assemblages in a multidimensional scaling analysis based on Bray-Curtis similarity of grouped microhabitat subsamples. Abundance values were log (x+1) transformed prior to analysis. Grey upward facing triangles represent sites of the upper (1-9) river reach; hollow circles represent sites (10-14) of the middle river reach and black downward facing triangles represent sites (15-20) of the lower reach of Bednja River. |
3.4 Odonata and microhabitat selection
The Cluster analysis (Fig. 4) of similarity between Odonata assemblages based on samples from different microhabitat groups showed grouping of the assemblages found on xylal (woody debris) substrates. Other samples showed no specific grouping with similar substrates. Samples with submerged macrophytes were removed from the analysis, as there was only one sample out of three recorded macrophyte microhabitats that had a Odonata assemblage present. Species specific contribution to similarity between same microhabitats and dissimilarity between different ones are shown in Table 3. Affiliations to different microhabitats are presented in Figure 5. Gomphus vulgatissimus was most abundant at microlithal substrate, Onychogomphus forcipatus, Platycnemis pennipes and Calopteryx virgo at xylal (woody debris) substrate (Fig. 5).
 |
Fig. 4 Position of samples taken on the longitudinal profile of Bednja River with regard to substrate composition in a Simprof test cluster analysis based on Bray-Curtis similarity of Odonata assemblages. Odonata abundance values (Log(x+1)) transformed prior to analysis. Substrate abbreviations: MESO = mesolithal (hand-sized cobbles), MICRO = microlithal (coarse gravel), PSA = psammal (sand), AKA = akal (fine to medium-sized gravel), XYL = xylal (woody debris), ARG = argyllal (clay), TECHNO = technolithal (artificial blocks). |
 |
Fig. 5 Number of Odonata nymph individuals per m2 for recorded species at different microhabitats in the Bednja River: (a) Gomphus vulgatissimus; (b) Onychogomphus forcipatus; (c) Platycnemis pennipes and (d) Calopteryx virgo. The abundances of Orthetrum albistylum were not graphically portrayed as they were found on one substrate subsample (macrophytes) solely. |
3.5 Odonata in relation to environmental variables
In the ‘Interactive-forward-selection’ of the CCA analysis, five of the sixteen environmental water parameters were statistically significant (oxygen saturation, pH, ammonium, water temperature and total nitrogen) when determining the variation of Odonata assemblages (Tab. 4). These five were later on processed in the final CCA analysis (Fig. 6).
Statistically significant parameters explained 45.4% of the total variation of Odonata distribution. The eigenvalues of the first two axes were 0.928 and 0.603. A Monte Carlo permutation test showed that the ordination was statistically significant (F = 5.8, p = 0.002). Juvenile Coenagrionidae were associated with sites with higher values of oxygen saturation. while Calopteryx virgo (Linnaeus, 1758) and juvenile Gomphidae with higher ammonium and total nitrogen concentrations. Platycnemis pennipes, Gomphus vulgatissimus (Linnaeus, 1758), juvenile Zygoptera and juvenile Libellulidae/Corduliidae were positioned relatively closely to each other, and associated with sites with lower ammonium concentrations, but no evident preference is visible.
Summary of environmental parameters analyzed in the CCA and their influence on Odonata variability. Significant variables (p < 0.05) are presented in bold.
 |
Fig. 6 CCA ordination of Odonata and environmental water properties. Odonata taxa are marked with black circles and environmental variables with arrows. Arrow lengths on the ordination show the relative importance of the explanatory variables (environmental properties), and their direction, relative to each other and to the sites, indicates positive or negative correlations. Environmental variable abbreviations are the following: Oxygen (%) = oxygen saturation; ∑N = total nitrogen (mg N/L); pH; Temp = water temperature (°C); NH4 + = ammonium concentration (mg N/L). Pla pen = Platycnemis pennipes; Gom vul = Gomphus vulgatissimus; Ony for = Onychogomphus forcipatus; Cal vir = Calopteryx virgo; Ort alb = Orthetrum albistylum. Coe. Gen sp. = Coenagrionidae juveniles, Gom. juvenile = Gomphidae juveniles; Zygop. juvenile = Zygoptera juveniles; Libel. juvenile = Libellulidae/ Corduliidae juveniles. |
3.6 Influence of hydromorphological modification on Odonata assemblages
No statistically significant response was shown when correlating Odonata species richness with hydromorphological modification scores. On the other hand, Odonata abundances were found to be strongly affected by modification of the river channel (R = −0.451; p < 0.05) where significant decreases of dragonfly abundances were observed with increased modifications to the river channel. The species-specific response curves against the mean score for the degree of hydromorphological modification is given in Figure 7.
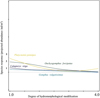 |
Fig. 7 Species response curves against the mean score for the degree of hydromorphological modification. |
4 Discussion
The studied lotic system, alike rivers of the Pannonian lowland ecoregion, is affected by primarily a combination of hydromorphological and water quality pressures (Schinegger et al., 2012). However, despite also having covered sites with high water quality and heterogenous habitat structures within this research, all identified nymphs were of mostly rheophile non threatened species (belonging to a conservation category of Least Concern; in the national and European red lists; Belančić et al., 2008; Kalkman et al., 2010). The absence of threatened and rare taxa from the assemblages could be accounted for by aspects not examined in this study such as terrestrial factors (e.g. Kadoya et al., 2008; Jonsen and Taylor, 2000; Nagy et al., 2019). Moreover, most of the lotic threatened European species have specific habitat requirements and very limited distribution range (Kalkman et al., 2008) which were not met in this study area. Vast areas of the Bednja River floodplain are used for intensive agriculture which could have replaced suitable vegetation structure for threatened adult species and consequently their aquatic nymphs (e.g. Hykel et al., 2016). Some studies showed that Anisoptera species tend to be more sensitive to landscape variables due to their higher dispersal ability, while Zygoptera species are more affected by instream local variables such as water surface cover by vegetation (Nagy et al., 2019).
The dominance of riverine species Platycnemis pennipes and Onychogomphus forcipatus within this study is not surprising and their abundance in anthropogenically impacted lotic systems within the same ecoregion has recently been reported by Vilenica et al. (2020a). Furthermore, lotic Calopteryx virgo and Gomphus vulgatissimus were also expected to inhabit the studied river. A single site record of the only lentic species, Orthetrum albistylum, was from a shallow site where absence of shading facilitates growth of reeds which obstruct flow providing conditions in line with the species ecology (Dijkstra and Lewington, 2006) but the record could also be considered accidental. Previous studies showed that this species favours man-made and anthropogenically altered habitats (Vilenica et al., 2011, 2020b).
The relatively low overall diversity could be accounted for by sampling effort. Although the 20 subsamples per site encompassed all present microhabitats and sampling was conducted in the recommended season for mid-sized rivers (AQEM consortium, 2002), the methodology does not encompass all Odonata life phases including exuviae and adults (Samways et al., 2009; Raebel et al., 2010) nor does it envisage collection of both spring and summer species (Corbet, 1999; Corbet et al., 2006). Expanding the research to different seasons could enable identification of those individuals which were at this time too juvenile and hence contribute to better knowledge of actual diversity and possibly confirm the presence/absence of rare or threatened species.
The increase in Odonata abundance with distance from source could be a result of the gradual increase in share of small-grained substrate as the river grows in size, providing more suitable habitats for the burrowing lifestyle of the Gomphidae taxa (Minshall, 1984; Suhling and Müller, 1996). The longitudinal increase in river size however was not followed by an increase in species richness as expected (e.g. Hawking and New, 1999; Oertli et al., 2002) most probably due to the relatively small total length of this river. Moreover, habitat heterogeneity has been compromised by hydromorphological impairments. Majority of the measured water quality parameters and nutrients increased along the longitudinal gradient which is expected (e.g. Harris, 2001) but the same is not the case with hydromorphological modification which is present to different extents also in the upper river reach. Historical and recent river regulations which have changed the natural channel dynamics (e.g. Elosegi et al., 2019) could be the rationale behind grouping of Odonata assemblages of upper reaches with middle and lower reach assemblages. The absence of Odonata nymphs from the most upstream study site near the river source is not completely surprising, as Odonata species richness in springs is generally lower compared to other river sections, due to their preference for higher water temperatures (Corbet and Brooks, 2008; Vilenica, 2017). Nevertheless, cold water lotic habitats and springs showed to be suitable for some species, such as Calopteryx virgo and Cordulegaster species (e.g. Lang et al., 2001; Vilenica et al., 2022), and we expect that with Odonata-targeted research, some of those could be recorded at the Bednja River spring. The recently regulated and reinforced study site 19 also lacked Odonata assemblages possibly due to unavailability of suitable habitats but also the tendency for predators to arrive late after habitat disturbances (Mackay, 1992).
Xylal (woody debris) which constituted only 9% of all sampled microhabitats accounted disproportionally for majority of the Zygoptera taxa collected within this study. In fact, exclusion of xylal from sampling would have resulted in Zygoptera to be entirely missing from six study sites. Calopterxy virgo has a known association to woody debris (e.g., Graf, 1997; Feld and Pusch, 1998) but Platycnemis pennipes and Onychogomphus forcipatus also had their highest abundance on such substrate. The reason for non-xylophagous taxa to be attracted to woody debris is because they seek prey associated with it (Hoffmann and Hering, 2000). Odonatan predation could also explain their high diversity on technolithal microhabitat at highly modified study site 17. It is possible that in this least mosaic habitat Odonata prey have little or no refuge spots when under predation pressure and are more easily caught. Apart from its habitat role for numerous aquatic invertebrates (Benke et al., 2003) the importance of large woody debris also extends to influencing flow patterns which shift sediment and as a result can increase heterogeneity at microhabitat scale (Elosegi et al., 2019).
Water quality parameters, besides ammonium and total nitrogen, had little influence on Odonata nymphs. Ammonium which is present in rivers as a result of untreated wastewater (EEA, 2018), in the studied lotic system has on average higher concentrations in the upper river reaches compared to downstream sites. We found Calopteryx virgo and juvenile Gomphidae to be associated with sites of somewhat higher ammonium and total nitrogen concentrations indicating their tolerance to water pollution. According to Vilenica (2017) Odonata could be associated with sites with slightly elevated nutrients or pollutants due to other favourable conditions such as more suitable microhabitat composition or higher prey availability. On the other hand, more species showed a negative association to ammonium. Although the adverse impact of ammonium on benthic macroinvertebrate abundance and richness has been documented (e.g. Laini et al., 2019) in the case with our studied lotic system ammonium concentrations were relatively low, ranging from below detection limit to 0.2612 mgN/L. It is possible the negative association to ammonium is a result of higher ammonium concentrations in the upstream studied sites, while Odonata displayed a downstream increase in abundance as previously discussed, and not species sensitivity to ammonium.
In our study, sites with higher water temperature were also reaches characterized by absence of shading from riparian vegetation. Riparian vegetation and trees not only influence water temperature (e.g. Garner et al., 2014) but are also the source of woody debris which has shown to be a favored odonatan microhabitat. Consequently, the negative association of species to water temperature might not be due to temperature but rather the lack of woody debris at these sites. The unshaded reaches are also associated with increased macrophyte presence and higher oxygen saturation.
The overall low Odonata species richness could have influenced our results that showed no significant correlations with hydromorphological modification scores. However, Odonata abundance, which had a greater gradient between study sites, was affected by modification of the river channel. The species-specific response curve indicated that all analysed Odonata species are sensitive to hydromorphological modification, with the possible exception of Platycnemis pennipes. As aforementioned, the reason could lay in the decreased habitat heterogeneity caused by river management practices. Carvalho et al. (2013) suggested assessing effects of habitat degradation on Odonata should be done at the suborder level as Anisoptera and Zygoptera respond differently to hydromorphological degradation.
The score for naturalness of the riparian zone, which is a constituent part of the EN 15843:2010 methodology also gave no significant correlations to species richness. The role of riparian vegetation for Odonata is unquestionable and has a history of being studied (e.g. Buchwald, 1992; Samways and Steytler, 1996). We found the EN 15843:2010 methodology to be unsuitable when it comes to Odonata as it measures departure from naturalness and not absence of vegetation, i.e. departure from what is expected for reference condition for given river type. For example, sites 2 and 17 had modified banks with grass which scores poorly by the assessment system, but none the less supported higher diversity than some sites with more natural bank vegetation. This is because Odonata taxa tend to be more sensitive to vegetation structure than composition (Smith et al., 2007).
5 Conclusion
Our study showed that Odonata nymphs often have specific preferences in terms of environmental conditions and habitat choice. This research once again stresses the significance of woody debris as an important microhabitat in river systems and the value of not removing it from river channels during river management practices. Since Odonata are an important insect order in conservation biology, these results showing their selection of woody debris as a microhabitat during their nymph phase has special significance. The impact of hydromorphological degradation also extends to Odonata as an insect order. Our results suggest that even species with a broad ecological tolerance can be sensitive to the impacts of hydromorphological degradation. As this is the first Odonata research at the studied lotic system, our results present an important basis for future trends, especially under the expected impacts of climate change.
Supplementary Material
Fig. S1. Singled out sites with highest Odonata species richness showing sampled microhabitat characteristics.
Tab. S1. Characteristics of the study sites on the Bednja River.
Tab. S2. Results for 16 individual scores for hydromorphological modification of study sites according to EN 15843:2010.
Access hereAcknowledgments
We thank all colleagues from University of Zagreb, Faculty of Science and Elektroprojekt who helped with field work. Emanuela Adrović is thanked for assisting with sorting of the collected material and Mladen Plantak is thanked for assisting with GIS analyses.
References
- American Public Health Association. 1992. Standard Methods for the Examination of Water and Wastewater, 18th ed. Washington, DC: APHA. [Google Scholar]
- Askew RR. 2004. The dragonflies of Europe. Second Edition, Harley Books, Essex: 308 p. [Google Scholar]
- AQEM consortium. 2002. Manual for the application of the AQEM method. A comprehensive method to assess European streams using benthic macroinvertebrates, developed for the purpose of the Water Framework Directive. Version 1.0, February 2002. [Google Scholar]
- Belančić A, Bogdanović T, Franković M, Ljuština M, Mihoković N, Vitas B. 2008. Crvena knjiga vretenaca Hrvatske, Ministarstvo kulture Republike Hrvatske, Ministarstvo zaštite okoliša i prirode, Državni zavod za zaštitu prirode, Zagreb, 132 p. [Google Scholar]
- Benke AC, Wallace JB. 2003. Influence of wood on invertebrate communities in streams and rivers. In: Gregory SV, Boyer KL and Gurnell AM (eds.), The ecology and management of wood in world rivers. American Fisheries Society, Symposium 37: Bethesda, Maryland. 149–177 p. [Google Scholar]
- Boudot JP, Kalkman VJ. 2015. Atlas of the Dragonflies and Damselflies of Europe. KNNV, Utrecht, 381 p. [Google Scholar]
- Bowler DE, Eichenberg D, Conze K-J, et al. 2021. Winners and losers over 35 years of dragonfly and damselfly distributional change in Germany. Divers Distrib 27: 1353–1366. [CrossRef] [Google Scholar]
- Bried JT, Herman BD, Ervin GN. 2007. Umbrella potential of plants and dragonflies for wetland conservation: a quantitative case study using the umbrella index. J Appl Ecol 44: 833–842. [CrossRef] [Google Scholar]
- Brochard C, Groendijk D, van der Ploeg E, Termaat T. 2012. Fotogids Larvenhuidjes van Libellen. Zeist: KNNV Publishers, 320 p. [Google Scholar]
- Buchwald R. 1992. Vegetation and dragonfly fauna – characteristics and examples of biocenological field studies. Vegetatio 101: 99–107. [Google Scholar]
- Carvalho FG, de Pinto NS, Oliveira Junior JMB, de Juen L. 2013. Effects of marginal vegetation removal on Odonata communities. Acta Limnol Bras 25: 10–18. [CrossRef] [Google Scholar]
- Céréghino R, Cugny P, Lavandier P. 2002. Influence of intermittent hydropeaking on the longitudinal zonation patterns of benthic invertebrates in a mountain stream. Int Rev Hydrobiol 87: 47–60. [CrossRef] [Google Scholar]
- Chovanec A, Waringer J, Raab R, Laister G. 2004. Lateral connectivity of a fragmented large river system: assessment on a macroscale by dragonfly surveys (Insecta: Odonata). Aquatic Conserv: Mar Freshw Ecosyst 14: 163–178. [CrossRef] [Google Scholar]
- Chovanec A, Schindler M, Waringer J, Wimmer R. 2015. The dragonfly association index (Insecta: Odonata) – a tool for the type-specific assessment of lowland river. River Res Appl 3: 627–638. [CrossRef] [Google Scholar]
- Clarke KR, Gorley RN. 2006. PRIMER V6: User Manual/Tutorial. Plymouth: Primer-E. [Google Scholar]
- Clausnitzer V. 2003. Dragonfly communities in coastal habitats in Kenya: indication of biotope quality and the need of conservation measures. Biodivers Conserv 412: 333–356. [CrossRef] [Google Scholar]
- Corbet PS. 1999. Dragonflies: Behaviour and Ecology of Odonata. Colchester: Harley Books. [Google Scholar]
- Corbet PS, Brooks S. 2008. Dragonflies, Collins New Naturalist Library series, Book 106. London: Harper Collins, 480 p. [Google Scholar]
- Corbet PS, Suhling F, Soendgerath D. 2006. Voltinism of Odonata: a review. Int J Odonatol 9: 1–44. [CrossRef] [Google Scholar]
- Čanjevac I. 2013. Typology of Discharge Regimes of Rivers in Croatia. Hrvat Geogr Glas 75: 23–42. [CrossRef] [Google Scholar]
- Dijkstra K-DB, Lewington, R. 2006. Field guide to the dragonflies of Britain and Europe. Gillingham: British Wildlife Publishing, 320 p. [Google Scholar]
- DIN EN. 2010. Water Quality – Guidance standard on determining the degree of modification of river hydromorphology, European standard CEN/TC 230 [Google Scholar]
- Dudgeon D. 2013. Anthropocene extinctions: global threats to riverine biodiversity and the tragedy of the freshwater commons. In: Sabater, S and Elosegi, A (eds.), River conservation: challenges and opportunities. Bilbao: Fundación BBVA, pp. 129–167. [Google Scholar]
- Elosegi A, Díez J, Mutz M. 2019. Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiology 657: 199–215. [Google Scholar]
- ESRI. 2010. ArcMap Version 10.0. ArcInfo license. Redlands, C.A. [Google Scholar]
- European Environment Agency. 2018. European waters – assessment of status and pressures. EEA Report 7/2018. Denmark, Copenhagen. [Google Scholar]
- Feld CK, Pusch M. 1998. Die Bedeutung von Totholzstrukturen für die Makroinvertebraten –Taxocoenosen in einem Flachlandfluß des Norddeutschen Tieflandes. Verh Westd Entom 1998: 165–172. [Google Scholar]
- Garner G, Malcolm IA, Sadler JP, Hannah DM. 2014. What causes cooling water temperature gradients in a forested stream reach? Hydrol Earth Syst Sci 18: 5361–5376. [CrossRef] [Google Scholar]
- Gerken B, Sternberg K. 1999. Die Exuvien Europaïscher Libellen, Arnika & Eisvogel, Hökster & Jena, 354 p. [Google Scholar]
- Giller PS, Malmqvist B. 1998. The Biology of Streams and Rivers. Biology of Habitats. New York: Oxford University Press, 304 p. [Google Scholar]
- Graf W. 1997. A new record of the perlid stonefly Agnetina elegantula (Klapalek, 1905) in Europe. In: Landolt, P and Sartori, M (eds.): Ephemeroptera and Plecoptera: Biology-Ecology-Systematics. Fribourg. 205–208 p. [Google Scholar]
- Harabiš F, Dolný A. 2010. Ecological factors determining the density-distribution of Central European dragonflies (Odonata). Eur J Entomol 107: 571–577. [CrossRef] [Google Scholar]
- Harabiš F, Dolný A. 2012. Human altered ecosystems: Suitable habitats as well as ecological traps for dragonflies (Odonata): the matter of scale. J Insect Conserv 16: 121–130. [CrossRef] [Google Scholar]
- Harris GP. 2001. Biogeochemistry of nitrogen and phosphorus in Australian catchments, rivers and estuaries: effects of land use and flow regulation and comparisons with global patterns. Mar Freshw Res 52: 139–149. [CrossRef] [Google Scholar]
- Hawking JH, New TR. 1999. The distribution patterns of dragonflies (Insecta: Odonata) along the Kiewa River, Australia, and their relevance in conservation assessment. Hydrobiology 392: 249–260. [CrossRef] [Google Scholar]
- Hoffman A, Hering D. 2000. Wood-associated macroinvertebrate fauna in central European streams. Int Rev Hydrobiol 85: 25–48. [CrossRef] [Google Scholar]
- Hykel M, Harabiš F, Dolný A. 2016. Assessment of the quality of the terrestrial habitat of the threatened dragonfl y, Sympetrum depressiusculum (Odonata: Libellulidae). Eur J Entomol 113: 476–481. [CrossRef] [Google Scholar]
- Jonsen I, Taylor PD. 2000. Calopteryx damselfly dispersions arising from multiscale responses to landscape structure. Conserv Ecol 4: 4. [Google Scholar]
- Kadoya T, Suda SI, Tsubaki Y, Washitani I. 2008. The sensitivity of dragonflies to landscape structure differs between life-history groups. Landsc Ecol 23: 149–158. [CrossRef] [Google Scholar]
- Kalkman VJ, Clausnitzer V, Dijkstra K-DB, Orr AG, Paulson DR, van Tol J. 2008. Global diversity of dragonflies (Odonata) in freshwater. In: Balian E, Martens K, Lévêque C and Segers H (eds.), A global assessment of animal diversity in freshwater. Hydrobiology 595: 351–363. [Google Scholar]
- Kalkman VJ, Boudot J-P, Bernard R, et al. 2010. European Red List of Dragonflies. Luxembourg: Publications Office of the European Union. [Google Scholar]
- Kalkman VJ, Boudot JP, Bernard R, De Knjif G, Suhling F, Termaat T. 2018. Diversity and conservation of European dragonflies and damselflies (Odonata). Hydrobiology 811: 269–282. [CrossRef] [Google Scholar]
- Laini A, Viaroli P, Bolpagni R, Cancellario T, Racchetti E, Guareschi S. 2019. Taxonomic and functional responses of benthic macroinvertebrate communities to hydrological and water quality variations in a heavily regulated river. Water 11: 1478. [CrossRef] [Google Scholar]
- Lang C, Müller H, Waringer JA. 2001. Larval habitats and longitudinal distribution patterns of Cordulegaster heros Theischinger and C. bidentata Sélys in an Austrian forest stream (Anisoptera: Cordulegastridae). Odonatologica 30: 395–409. [Google Scholar]
- Mackay RJ. 1992. Colonization by lotic macroinvertebrates: a review of processes and patterns. Can J Fish Aquat Sci 49: 617–628. [CrossRef] [Google Scholar]
- Minshall GW. 1984. Aquatic insect–substratum relationship. In: Resh VH and Rosenberg DM (eds.), The ecology of aquatic insects. New York: Praeger Scientific, 358–400 p. [Google Scholar]
- Moore NW. 1997. Status survey and conservation action plan. Dragonflies. IUCN/SSC Odonata Specialist Group. IUCN, Gland and Cambridge, 28 p. [Google Scholar]
- Nagy HB, László Z, Szabó F, Szöcs L, Dévai G, Tóthmérész B. 2019. Landscape-scale terrestrial factors are also vital in shaping Odonata assemblages of watercourses. Sci Rep 9: 18196. [CrossRef] [PubMed] [Google Scholar]
- Oertli B, Joye DA, Castella E, Juge R, Cambin D, Lachavanne JB. 2002. Does size matter? The relationship between pond area and biodiversity. Biol Conserv 104: 59–70. [CrossRef] [Google Scholar]
- Ott J. 2010. Dragonflies and climatic changes – recent trends in Germany and Europe. BioRisk 5: 253–286. [CrossRef] [Google Scholar]
- Poff NL, Zimmermann JKH. 2010. Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshw Biol 55: 194–205. [CrossRef] [Google Scholar]
- Raebel EM, Merckx T, Riordan P, Macdonald DW, Thompson DJ. 2010. The dragonfly delusion: why it is essential to sample exuviae to avoid biased surveys. J Insect Conserv 14: 523–534. [CrossRef] [Google Scholar]
- Rouquette JR, Thompson DJ. 2005. Habitat associations of the endangered damselfly, Coenagrion mercuriale, in a water meadow ditch system in southern England. Biol Conserv 123: 225–235. [CrossRef] [Google Scholar]
- Samways MJ, McGeoch MA, New TR. 2009. Insect conservation: handbook of approaches and methods. Oxford: Oxford University Press, 432 p. [Google Scholar]
- Samways MJ, Steytler NS. 1996. Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biol Conserv 78: 279–288. [CrossRef] [Google Scholar]
- Schinegger R, Trautwein C, Melcher A, Schmutz S. 2012. Multiple human pressures and their spatial patterns in European running waters. Water Environ J 26: 261–273. [CrossRef] [PubMed] [Google Scholar]
- Simaika JP, Samways MJ. 2008. Valuing dragonflies as service providers. In: Cordoba-Aguilar, A (eds.), Dragonflies: Model Organisms for Ecological and Evolutionary Research. Oxford: Oxford University Press, 109–123. [CrossRef] [Google Scholar]
- Smith J, Samways ML, Taylor S. 2007. Assessing riparian quality using two complementary sets of bioindicators. Biodivers Conserv 16: 2695–2713. [CrossRef] [Google Scholar]
- Strayer DL. 2006. Challenges for freshwater invertebrate conservation. J N Am Benthol Soc 25: 271–287. [CrossRef] [Google Scholar]
- Suh AN, Samways AJ. 2005. Significance and temporal changes when designing a reservoir for conservation of dragonfly diversity. Biodivers Conserv 14: 165–178. [CrossRef] [Google Scholar]
- Suhling F, Müller O. 1996. Die Flussjungfern Europas. Gomphidae. Westarp Wissenschaften / Die Neue Brehm-Bücherei (Bd. 628), Magdeburg. [Google Scholar]
- Suhling F, Sàhlen G, Martens A, Marais E, Schütte C. 2006. Dragonfly assemblages in arid tropical environments: a case study from western Namibia. Biodivers Conserv 15: 311–332. [CrossRef] [Google Scholar]
- Šegota T, Filipčić A. 2003. Köppenova podjela klima i hrvatsko nazivlje, Geoadria 8: 17–37. [Google Scholar]
- Ter Braak CJF, Šmilauer P. 2012. Canoco reference manual and user’s guide: software for ordination, version 5.0, Ithaca USA: Microcomputer Power, 496 [Google Scholar]
- Termaat T, van Strien AJ, van Grunsven RHA, et al. 2019. Distribution trends of European dragonflies under climate change. Divers Distrib 25: 936–950. [CrossRef] [Google Scholar]
- TIBCO Software Inc. 2017. Statistica (data analysis software system), version 13. Retrieved from: http://statistica.io. [Google Scholar]
- Vilenica M, Mičetić Stanković V, Franković M. 2011. Dragonfly fauna (Insecta, Odonata) in the Turopolje region (Croatia). Nat Croat 20: 141–158. [Google Scholar]
- Vilenica M. 2017. Ecological traits of dragonfly (Odonata) assemblages along an oligotrophic Dinaric karst hydrosystem. Ann Limnol - Int J Lim 53: 377–389. [CrossRef] [EDP Sciences] [Google Scholar]
- Vilenica M, Kerovec M, Pozojević I, Mihaljević Z. 2020a. Odonata assemblages in anthropogenically impacted lotic habitats. J Limnol 80: 1968. [CrossRef] [Google Scholar]
- Vilenica M, Pozojević I, Vučković N, Mihaljević Z. 2020b. How suitable are man-made water bodies as habitats for Odonata? Knowl Manag Aquat Ecosyst 421: 13. [CrossRef] [EDP Sciences] [Google Scholar]
- Vilenica M, Rebrina F, Matoničkin Kepčija R, et al. 2022. Aquatic macrophyte vegetation promotes taxonomic and functional diversity of Odonata assemblages in intermittent Karst rivers in the Mediterranean. Diversity 14: 31. [CrossRef] [Google Scholar]
- Villalobos-Jiménez G, Dunn AM, Hassall C. 2016. Dragonflies and damselflies (odonata) in urban ecosystems: a review. Eur J Entomol 113: 217–232. [CrossRef] [Google Scholar]
- Ward JV, Stanford JA. 1979. Ecology of regulated streams. New York: Plenum Press. 398 p. [Google Scholar]
- Zwick P. 1992. Stream habitat fragmentation - a threat to biodiversity. Biodivers Conserv 1: 80–97. [CrossRef] [Google Scholar]
Cite this article as: Vidaković Maodus I, Pozojević I, Vilenica M, Mihaljević Z. 2022. Longitudinal dynamics of Odonata assemblages in an anthropogenically impacted lotic system. Int. J. Lim. 58: 7
All Tables
Summary of environmental variables at the 20 study sites given as range of measured values.
Odonata abundance given as number of individuals per meter squared. Only study sites and microhabitats where nymphs were recorded are given. Microhabitat abbreviations: MESO = mesolithal (hand-sized cobbles); MICRO = microlithal (coarse gravel); AKA = akal (fine to medium-sized gravel); PSA = psammal (sand); XYL = xylal (woody debris); ARG = argyllal (clay); TECHNO = technolithal (artificial blocks); MACROP = both submerged and emergent macrophytes. * - juvenile instars not identified to species level.
Summary of environmental parameters analyzed in the CCA and their influence on Odonata variability. Significant variables (p < 0.05) are presented in bold.
All Figures
 |
Fig. 1 Position of the Bednja River Catchment in Croatia and distribution of the 20 study sites along the Bednja River with respect to altitude. |
| In the text | |
 |
Fig. 2 Hydromorphological modification at each study site according to norm EN 15843:2010. The mean score is derived from 16 individual features assessed. Grouped scores are given for modification of river channel, banks and riparian zone and floodplain. Individual score is given only for vegetation type / structure on banks and adjacent land. |
| In the text | |
 |
Fig. 3 Position of the study site Odonata assemblages in a multidimensional scaling analysis based on Bray-Curtis similarity of grouped microhabitat subsamples. Abundance values were log (x+1) transformed prior to analysis. Grey upward facing triangles represent sites of the upper (1-9) river reach; hollow circles represent sites (10-14) of the middle river reach and black downward facing triangles represent sites (15-20) of the lower reach of Bednja River. |
| In the text | |
 |
Fig. 4 Position of samples taken on the longitudinal profile of Bednja River with regard to substrate composition in a Simprof test cluster analysis based on Bray-Curtis similarity of Odonata assemblages. Odonata abundance values (Log(x+1)) transformed prior to analysis. Substrate abbreviations: MESO = mesolithal (hand-sized cobbles), MICRO = microlithal (coarse gravel), PSA = psammal (sand), AKA = akal (fine to medium-sized gravel), XYL = xylal (woody debris), ARG = argyllal (clay), TECHNO = technolithal (artificial blocks). |
| In the text | |
 |
Fig. 5 Number of Odonata nymph individuals per m2 for recorded species at different microhabitats in the Bednja River: (a) Gomphus vulgatissimus; (b) Onychogomphus forcipatus; (c) Platycnemis pennipes and (d) Calopteryx virgo. The abundances of Orthetrum albistylum were not graphically portrayed as they were found on one substrate subsample (macrophytes) solely. |
| In the text | |
 |
Fig. 6 CCA ordination of Odonata and environmental water properties. Odonata taxa are marked with black circles and environmental variables with arrows. Arrow lengths on the ordination show the relative importance of the explanatory variables (environmental properties), and their direction, relative to each other and to the sites, indicates positive or negative correlations. Environmental variable abbreviations are the following: Oxygen (%) = oxygen saturation; ∑N = total nitrogen (mg N/L); pH; Temp = water temperature (°C); NH4 + = ammonium concentration (mg N/L). Pla pen = Platycnemis pennipes; Gom vul = Gomphus vulgatissimus; Ony for = Onychogomphus forcipatus; Cal vir = Calopteryx virgo; Ort alb = Orthetrum albistylum. Coe. Gen sp. = Coenagrionidae juveniles, Gom. juvenile = Gomphidae juveniles; Zygop. juvenile = Zygoptera juveniles; Libel. juvenile = Libellulidae/ Corduliidae juveniles. |
| In the text | |
 |
Fig. 7 Species response curves against the mean score for the degree of hydromorphological modification. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


