| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 56, 2020
|
|
|---|---|---|
| Article Number | 24 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/limn/2020022 | |
| Published online | 25 September 2020 | |
Research Article
Limnological characteristics, community metabolism and management strategies of a coastal sinkhole in Cuba (Cenote Jennifer)
1
Centro de Estudios Geomáticos, Ambientales y Marinos (GEOMAR), Avenida Ejército Nacional 804, Polanco V Sección, Miguel Hidalgo, Ciudad de México CP: 11560, Mexico
2
Departamento de Hidráulica, Facultad de Ciencias Técnicas, Universidad de Ciego de Ávila, Carretera a Morón, Km 9, Ciego de Ávila, Cuba
3
Maestría en Calidad Alimentaria, Universidad Politécnica de Madrid, Avenida Puerta de Hierro, 2, 28040 Madrid, Spain
4
School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332-0100, USA
5
Centro Meteorológico Provincial Ciego de Ávila, Avenida de los Deportes S/N, Ciego de Ávila CP: 65100, Cuba
6
Departamento de Turismo y Organización Empresarial, Facultad de Ciencias Económicas y Empresariales, Economía y Gestión Empresarial, Universidad de Ciego de Ávila, Carretera a Morón, Km 9, Ciego de Ávila, Cuba
* Corresponding author: coraleslhf@gmail.com
Received:
24
May
2020
Accepted:
2
September
2020
The Cenote Jennifer is an important and unique aquatic sinkhole in Cayo Coco (Jardines del Rey Tourist Destination) that has brackish to saline water. Two samplings were made in 1998 and 2009, and 4 metabolism community experiments in 2009. Some limnological parameters were measured in both samplings (temperature, salinity, pH, dissolved oxygen major ions, hydrogen sulfide, nutrients and others). Community metabolism was measured through incubated oxygen concentration in clear and dark oxygen bottles. Results showed that the sinkhole limnology depends on rainfall and light incidence year, with some stratification episodes, due to halocline or oxycline presence, rather than thermocline. The sinkhole water was oligotrophic (total nitrogen of 41.5 ± 22.2 μmol l−1 and total phosphorus of 0.3 ± 0.2 μmol l−1) and with low productivity (gross primary productivity of 63.0 mg C m−2 d−1). Anoxia and hypoxia were present at the bottom with higher levels of hydrogen sulfide, lower pH and restricted influence of the adjacent sea (2 km away). To protect the Cenote Jennifer, tourist exploitation should be avoided and more resources to ecological and morphological studies should be allocated, and eventually use this aquatic system only for specialized diving. For conservation purposes, illegal garbage disposal in the surrounding forest should end.
Key words: Limnological / metabolism / management / sinkhole / Cuba
© EDP Sciences, 2020
1 Introduction
Approximately 15 percent of the Earth's land surface is karst. The distribution of karst is essentially the same as the distribution of carbonate rocks, which means that karst terrain occurs mostly in the great sedimentary basins of the world. Karst occurs in North America and the Caribbean region, and is well represented in the Greater Caribbean islands like Hispaniola, Jamaica, Puerto Rico and The Bahamas. It is also well represented in the Yucatan Peninsula (Mexico), in Florida and in the Mississippi basins (USA). In Cuba, karst represents 66% of its total area, including most of the smaller islands and keys of the archipelago (Gutiérrez-Domech, 1998). One of the karstic mesoforms are anchialine caves, partially or totally submerged, located within a few kilometers inland in volcanic or karstic limestone terrain, named sinkholes. Such sinkholes are locally termed “cenotes” in the Yucatan Peninsula in Mexico, “blue holes” in The Bahamas and Belize, and “grietas” in the Galapagos Islands (Iliffe and Kornicker, 2009). In some parts of Cuba, these caves are known as “cenotes”, “dolinas” or “lagunas”.
Around the world, sinkholes have been well studied, particularly in the Yucatan Peninsula (México) (Suárez Morales and Rivera Arriaga, 1998; Schmitter-Soto et al., 2002; Beddows et al., 2007; Sánchez y Pinto et al., 2015; Cervantes-Martínez et al., 2018; Enseñat-Soberanis et al., 2019), Australia (Humphreys, 1999, Humphreys et al., 1999; Somaratne, 2017), USA (Brinkmann et al., 2008; Emmert 2016; Young et al., 2018) and The Bahamas (Gonzalez et al., 2011; Keeton, 2017; Tamalavage et al., 2018). Brankovits et al. (2017) studied the role of coastal karts subterranean estuaries as sink of methane and its contribution of nutrients and carbon to the ocean.
In Cuba, sinkholes have been poorly studied and the focus has been on biodiversity (Holthuis, 1974; Silva Taboada, 1974; García Debrás et al., 1997; García-Machado et al., 2011; Reynaldo et al., 2016; Pérez-García et al., 2018), morphology (Guarch Rodríguez and Corella Varona, 2010), saline intrusion (Salomon, 2019) and fecal contamination (Mulec and Oarga, 2014).
The geology of Cayo Coco consists of carbonate rocks of the upper-middle Pleistocene (calcarenites and biocalcarenites), belonging to the Jaimanitas formation (Iturralde‐Vinent, 1994). There are many karstic forms, but only one sinkhole is known (Cenote Jennifer).
There are only two references to the Cenote Jennifer (G'meiner, 2016; Peros et al., 2017), both regarding paleolimnological results from a sediment core, but they were the first to show some limnological parameters (salinity, temperature, dissolved oxygen and pH); the sinkhole area and bathymetry were also described by these authors. This sinkhole was also studied in 1998 and 2009, mainly to determine limnological parameters. Cayo Coco is located at the Jardines del Rey tourist destination, where the creation of new products like nature tourism, ecotourism and health tourism, is essential for economic success.
The objective of this work is to analyze some limnological parameters and primary productivity of the Cenote Jennifer, and based on such results; provide conservation and management considerations for tourist and scientific use.
2 Materials and methods
2.1 Study area
The geology of Cayo Coco consists of carbonate rocks of the upper-middle Pleistocene (calcarenites and biocalcarenites), belonging to the Jaimanitas formation (Iturralde‐Vinent, 1994). Cayo Coco has been tectonically stable throughout the Holocene (Iturralde‐Vinent, 1994). Most of the soils of Cayo Coco are shallow and poorly developed on a limestone substrate (Alcolado et al., 2007). The study site (Cenote Jennifer), is located in exposed limestone on the northeast of Cayo Coco, at approximately 2 Km from the coast (Fig. 1).
In Cayo Coco, dry conditions prevail from December to April (<60.0 mm rain per month), and wetter conditions (>100.0 mm rain per month) from May to November, with a marked mid-summer drought (MSD) in July-August. Monthly average temperature ranges from 23.0 °C in January to 29.0 °C in July (Batista Tamayo et al., 2006).
Historically, Cayo Coco has been virtually devoid of human population. Social and economic intervention began at the beginning of the 20th century. It was characterized mainly by forest exploitation and charcoal production; in the adjacent coast, fishing was the principal activity. Cattle ranching was also tried for some time, which is why feral cattle still inhabit Cayo Coco. Many forest areas were cleared for timber harvesting or burned for pasture lands (Alcolado et al., 1998). In most cleared areas, secondary forests have developed. In the early 1980s several studies to assess the potential use of the area for tourism were conducted. In 1980, the construction of roads on the island began and in the summer of 1986, the construction of a causeway started. It was completed by 1988. For the first time, Cayo Coco was connected to the mainland and it made possible the development of tourism infrastructure. In 1990, the first master plan for the development of the area was implemented. By 1993, a large tourist complex had been built on the northeast shore of Cayo Coco (1.5 km from the Cenote Jennifer) and a nearby second hotel was completed and opened on December 20, 1996. By 2016, almost 4000 rooms for tourism only in Cayo Coco and more than 5000 rooms in the province of Ciego de Ávila had been built (ONEI, 2018).
Two decades before, tourism authorities in search for nature tourism options, had designed a trail named “Las Dolinas”, where the Cenote Jennifer was included as the main attraction, but but it never yielded the expected results.
The Cenote Jennifer is located (22°31′50″ N, 78°22′58″ W) in Cayo Coco, province of Ciego de Ávila, Cuba (Fig. 1). It has a surface area of approximately 400 m2 (0.04 ha) and a depth of 17 m from the highest point of the surrounding rock surface to the sediment-water interface. Depth ranges from 9 m near the edge of the sinkhole to 15 m at the center (G'meiner, 2016).
The Cenote Jennifer can be classified as a typical sinkhole in the form of a glass (variant form with attached flooded galleries), according to classification given by Hall (1936) and as a sinkhole in a coastal line, according to the criteria of Navarro-Mendoza (1988).
The dense forest directly surrounding the sinkhole is characterized as a thorny limestone shrub wood, consisting of Picrodendron baccatum (Jamaican walnut), and Bursera simaruba (gumbo-limbo) trees and a shrub layer of Buxus spp., Randia spp. and Croton spp.. Approximately 50 m to the north of the Cenote Jennifer is a shallow hypersaline lagoon (Pupi II), fringed by Avicennia germinans and Conocarpus erectus (G'meiner, 2016).
The direction of the groundwater flow in Cayo Coco is quite variable, usually radial, with from points of greater dimensions towards its central zone. The absolute levels of water vary from 0.11 to 0.57 meters in the wet period and from 0.04 to 0.44 m during the dry season, except in areas of possible tectonic dislocation, where static underground water levels are below average sea level.
The wastewater treatment system (stabilization lagoons) of Cayo Coco is near the sinkhole (800 m) (Fig. 1). It has effluent infiltration to the ground as tertiary and final treatment. This final effluent disposal dates from the beginning of the present century and could affect groundwater in Cayo Coco
 |
Fig. 1 Location figures and partial view of Cenote Jennifer, Cayo Coco, Cuba. |
2.2 Sampling and analysis
Two separate samplings were made at the sinkhole. The first sampling took place in January and February of 1998. Samples (4) were taken from the surface and at depths of 4 m, 8 m and 12 m. Temperature, pH, and salinity were measured in situ (with an ORION probe) and samples for dissolved oxygen, nutrients (ammonium), total alkalinity, total hardness, carbon dioxide, hydrogen sulphide, some ions (calcium, magnesium, potassium, chloride and sodium), and soluble reactive silicate (SRSi) were taken to the laboratory. All samples were preserved according to the methodology for each parameter. The methodologies proposed by AHPA (1985) for each parameter were used in the laboratory. Major ions were determined by Atomic Absorption Spectrometry (K+: DL = 0.1 mg/L; Ca2+: DL = 0.002 mg/L; Mg2+: DL = 0.001 mg/L; Na1+: DL = 0.1 mg/L), total hardness by EDTA titration (DL = 0.1 mg/L), total alkalinity by titration with HCL (0.1 mg/L), hydrogen sulfide by iodometric method (DL = 0.1 mg/L) and chloride by argentometric method (DL= 0.1 mg/L). Dissolved oxygen was determined by Winkler method (DL = 0.1 mg/L). Ammonium was determined with automatic method (phenate method, DL = 0.14 μM/L) R and SRSi were determined automatically through molybdate method (DL = 0.01 μM/L) and total nitrogen and phosphorus was determined by persulfate reduction (DL = 0.42 μM/L and 0.02 μM/L, respectively).
Four samplings were made in March (12 and 19) and in April of 2009 (28 and 30). Surface water temperature and salinity (with digital thermo-salinometer WLW), dissolved inorganic nutrients (ammonium, nitrate + nitrite), total nutrients (nitrogen and phosphorus) and soluble reactive silicate (SRSi) were measured before and after every metabolism experiment. All samples were preserved using the methodology for each parameter. The methodologies proposed by AHPA (1985) for each parameter were used in the laboratory. Dissolved oxygen was determined by Winkler method (DL = 0.1 mg/L). Nitrate + nitrite through automatic method (cadmium reduction technique, DL = 0.42 μM/L) and soluble reactive phosphorus by molybdate method (DL = 0.02 μM/L). Ammonium was determined with automatic method (phenate method, DL = 0.14 μM/L) R and SRSi were determined automatically through molybdate method (DL = 0.01 μM/L) and Total Nitrogen and Phosphorus were determined by persulfate reduction (DL = 0.42 μM/L and 0.02 μM/L, respectively)
Community metabolism was measured by evaluating the evolution of oxygen dynamics, using the light and dark bottles incubation method. The bottles were incubated for four to six light-hours at depths of 0, 1, 3, 5, 7, 9 and 11 m. At each depth, nine oxygen bottles were filled (three for initial oxygen determination, three for light incubation and three for dark incubation). While sampling, extreme precautions were taken to completely avoid bubbling that could alter oxygen content, as recommended by Valdespino-Castillo et al. (2014). Dissolved oxygen concentration in each bottle was determined in the laboratory, in triplicate for each sample bottle to minimize and assess error (see Valdespino-Castillo et al., 2014 for further details on the method). To determine dissolved oxygen concentration (DO), the method of Winkler, modified by Carritt and Carpenter (UNESCO, 1983) was used.
2.3 Metabolism calculation
Gross primary production (GPP), net primary production (NPP), and community respiration (R) were calculated using the oxygen change rate in the light and dark bottles, respectively, following Wetzel and Likens (1991), and therefore thereafter dividing the differences between initial and final oxygen concentrations by the specific incubation time of each set of bottles.
Conversion of oxygen rates to carbon rates was performed with the theoretical and most widely used conversion values PQ = 1.3 and RQ = 1.0 (Gazeau et al., 2005).
2.4 Density of use
To estimate the maximum density of use (MDU), the total public use area of the sinkhole was divided by 4 m2, which is the estimated vital area (VA) for a visitor to feel comfortable in recreational spaces (García Hernández, 2001)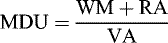 (1)where MDU (Maximum density of use); WM (Water mirror); RA (Rest area); VA (Vital area).
(1)where MDU (Maximum density of use); WM (Water mirror); RA (Rest area); VA (Vital area).
3 Results
3.1 Limnological behavior
All mean values of measured parameters, correlation and ANOVA results in the Cenote Jennifer (in 1998 and 2009) are shown in Table 1. PCA analysis (for measured parameters in 1998) shows four distinct layers; surface layer, first layer (4 m), medium layer (8 m) and bottom and anoxic layers (Fig. 2).
Mean values of each measured parameter per depth in Cenote Jennifer in 1998 and 2009. Minor bold letters denote marked correlation among parameters. Major letters denote significant differences (p < 0.05) only in surface samples among samplings.
 |
Fig. 2 PCA analysis for all measured parameters in Cenote Jennifer in 1998. |
3.1.1 Temperature and salinity
Mean temperature was higher at surface (F = 160.44, p < 0.05) and declined as depth decreased, but it was not clear thermocline and the difference between surface temperature and temperature at 12 m was only of 1.9 °C in 1998 (Fig. 3A). Temperature was different for all samplings (in 1998 and 2009), with the highest value at surface in 1998 (29.6 °C).
Surface mean salinity was lower (F = 40.73, p < 0.05) than at 4, 8 and 12 m; however between 4 and 12 m, salinities were similar (between 12.5 and 15.0) without significant differences in 1998. Halocline was found in the first 4 m of depth, varying around five (5.0) units between surface and 4 m in 1998 (Fig. 3B). The lowest salinity was at surface in 1998 sampling (Tab. 1) and was significant different for all samplings.
 |
Fig. 3 Vertical profiles of (A) temperature (°C), (B) salinity, (C) pH and (D) dissolved oxygen (mg/L) in cenote Jennifer in 1998 samplings. |
3.1.2 Hydrogen ion concentration (pH) and dissolved oxygen (DO)
Mean pH was higher (F = 154.99, p < 0.05) in surface water (8.08 ± 0.01) and declined (significantly) for each sampled depth in 1998 (Tab. 1; Fig. 3C).
DO profile showed a clear oxycline, which began between 4 and 8 m, probably at 6 m, because some occasional measurements showed Secchi transparency of 6–8 m. At surface, mean DO was 7.2 ± 0.2 mg l−1 (Tab. 1; Fig. 3D), while at 8 m was 5.7 ± 0.2 mg l−1 and there was anoxia at 12 m in 1998. All samplings (in 1998 and 2009) were significantly different, with the highest value at surface (10.3 mg l−1) in the sampling of March 2 (Tab. 1). There was hypoxia at 9 m and at 1 m in the second sampling of April (Fig. 4).
 |
Fig. 4 Vertical profile of temperature (°C) in cenote Jennifer in 2009 samplings. |
3.1.3 Hydrogen sulphide (H2S) and sulfate (SO42−)
Mean hydrogen sulphide was higher (F = 1205.85, p < 0.05) at 12 m and increased from 150.0 ± 2.0 mg l−1 at surface to 207.9 ± 3.4 mg l−1 at 12 m in 1998 (Tab. 1). Sulfate showed a more significant decrease than hydrogen sulphide, which dropped sharply from 5337.2 ± 129.1 mg l−1 at 4 m to 4135.3 ± 430.6 mg l−1 at 12 m (Tab. 1).
3.1.4 Dissolved inorganic nitrogen (ammonium)
This nitrogen form increased significantly (F = 130.06, p < 0.05) from 2.9 ± 0.05 μmol l−1 at surface and 4 m to 7.9 ± 0.09 μmol l−1 at 12 m in 1998. Ammonium had significant (positive and negative) correlations with most measured parameters (Tab. 1) in 1998, but this behavior was different in the 2009 samplings. Only the samplings of April 2009 were similar and with the highest value of ammonium at surface (4.5 μmol l−1) was recorded in the first sampling of 2009 (March 1) (Tab. 1).
3.1.5 Soluble reactive silicate (SRSi)
Mean SRSi had the highest concentration at 12 m (231.8 ±0.3 μmol l−1), increasing from surface 104.8 ± 0.3 μmol l−1 to 231.8 ± 0.3 μmol l−1 in 1998. The highest SRSi concentration at surface was in 1998 and only the samplings of April of 2009 were similar (Tab. 1). These concentrations are higher than those reported for seawater (less than 3.0 μmol l−1) and are normal for groundwater.
3.1.6 Major ions
Major ions (in meq l−1) showed the following order: Cl− > Na+ − > Mg2+ > SO42− > Ca2+ > K+. All major ions had significant correlation among them (Tab. 1). K+, Mg2+, Na+ and Cl− increased significantly from surface to 12 m; while Ca2+ and SO42− decreased with depth (Tab. 1).
3.1.7 Total hardness and alkalinity
Total hardness increased significantly from surface to 12 m and had a significant correlation with total alkalinity, ammonium and major ions. Total alkalinity showed a pattern similar to that of total hardness, increasing from surface to 12 m, and with significant correlation with ammonium and major ions (Tab 1).
3.1.8 Dissolved inorganic nitrogen (DIN)
All dissolved inorganic nitrogen forms were measured in 2009; ammonium was the principal fraction of DIN (more than 80% for all samplings). Mean nitrate + nitrite concentration was higher (F = 10.17, p < 0.05) in March (0.7 ± 0.13 μmol l−1) samplings than in April samplings (0.5 ± 0.063 μmol l−1) (Tab. 1). Mean DIN concentration was (4.7 ± 0.53 μmol l−1), with higher (F = 6.45, p < 0.05) mean concentration in March than in April. DIN showed a significant correlation only with ammonium in 2009 (Tab. 1).
3.1.9 Total nitrogen (TN)
Most nitrogen in the sinkhole was in organic form, DIN only represented between 7 and 21% of TN. Mean TN concentration was 41.5 ± 22.23 μmol l−1 for all the samplings, with greatest concentration in the first sampling of 2009 (Tab. 1).
3.1.10 Soluble reactive phosphorus (SRP) and total phosphorus (TP)
Most TP was organic. SRP only represented between 0 and 40% of TP. Mean SRP was 0.07 ± 0.063 μmol l−1, with undetectable concentration in the second sampling of March of 2009 (Tab. 1). Mean TP was 0.3 ± 0.23 μmol l−1 for all the sampling period (Tab. 1).
3.2 Community metabolism
Vertical profiles of gross primary production (GPP), respiration (R) and Net primary production (NPP) in mg O2 m−3 h−1 are shown in Figure 5. Mean production (both net and gross) rates were different for each sampling; however, there were no significant differences among experiments. In the first experiment of March (0.29 mg O2 m−3 h−1) and in the second of April (0.21 mg O2 m−3 h−1), maximum GPP was documented at 5 m, while in the second experiment of March (0.61 mg O2 m−3 h−1) it was found at 1 m. In the first experiment of April (0.37 mg O2 m−3 h−1), maximum GPP was found at surface. Maximum mean GPP (for all experiments), was of 0.29 mg O2 m−3 h−1 at 1 m. NPP behavior was similar to that of GPP for each experiment, except for the second experiment of April (0.14 mg O2 m−3 h−1) with the maximum at 11 m and maximum mean NPP was found at surface (0.14 mg O2 m−3 h−1). Respiration also had a vertical gradient, and maximum for most experiments was found at 5 m. Only in the first experiment of April (0.35 mg O2 m−3 h−1) the maximum was recorded at 1 m. Mean R was 0.20 mg O2 m−3 h−1 at 5 m.
After vertically integrating the whole production layer, including experiments at all depths, we calculated vertically integrated metabolic rates (Tab. 2). For each experiment month, values of integrated metabolic rates were similar, without significant differences.
In terms of carbon fluxes, GPP had a mean value of 63.0 mg C m−2 d−1 and mean NPP was 23.0 mg C m−2 d−1. The potential carbon exportation from the production layer through biomass sinking (f = NPP/GPP) was 37% in the Cenote Jennifer. Mean R for all experiments was 31.0 mg C m−2 d−1. The ratio PB:R = 2.0 showed that the prevailing processes at the sinkhole are autotrophic rather than completely heterotrophic.
 |
Fig. 5 Vertical profiles of (A) GPP, (B) NPP and (C) R (in mg O2 m−3 h−1) in Cenote Jennifer in 2009 experiments. |
Integrated metabolic rates (vertically) in Cenote Jennifer in 2009 experiments.
4 Discussion
4.1 Limnological parameters and community metabolism
Cenote Jennifer has a circular shape and is an open sinkhole according to the classification proposed by Hall (1936). It is a small sinkhole (< 1 ha) that may be unique in the Cayo Coco area. Cenote Jennifer could be classified as a lentic sinkhole according to Beddows et al. (2006). There is not national inventory of large, medium or small sinkholes in Cuba, but it is known that these aquatic systems are distributed throughout the country (including the main and smaller islands of the archipelago). Some authors state that the principal areas of sinkhole are located in the karstic zones of Pinar del Río, the Zapata Swamp and south of Isla de la Juventud (Núñez Jiménez et al., 1968; Franco and De la Torre, 1980; Gutiérrez-Domech, 1998), but the presence of sinkhole have been reported in Holguín (Echtinger, 2000; Guarch and Corella, 2010) and in karstic zones of Havana and Central Cuba (Gutiérrez-Domech, 1998).
Vertically, the sinkhole did not show a clear thermocline between surface and 12 m (variation of 1.9 °C) in 1998 and showed similar behavior as observed by Peros et al. (2017), variation of 4.0 °C between surface and 14 m. The small difference between samplings (1998 and 2014) could be due to the sampling date (February in 1998, dry and cold season) and in 2017, sampling was performed in July, when mean air temperature is higher in Cayo Coco (wet and warm season). Similar behavior was observed at the Cenote Tanque Azul, in the province of Holguín; with a temperature variation of 3.0 °C of thermocline between surface and 41 m (Echtinger, 2000). Cervantes-Martínez et al. (2002) found mixed results with thermal stratification, but slightly or no temperature differences in the water column in eight sinkholes of the Yucatan Peninsula. The presence of thermocline was found by Herrera-Silveira and Comín (2000) in lotic and lentic sinkholes during the dry and rainy seasons, while the water column remained mixed during the winter storm season in Yucatán. One factor that could explain the lack of a clear thermocline (even in the warm season) at the Cenote Jennifer is its location. It is surrounded by a dense forest, which limits the direct incidence of the sun light, which results in less transmission of radiant heat between the atmosphere and its waters (Díaz–Arce et al., 2001; Schmitter-Soto et al., 2002). The thermal stratification process in lakes, for example, depends of diverse factor as wind influence, water movements and lake form (Wetzel, 2001), so, Cenote Jennifer is protected (by a dense forest) from winds and direct light incidence during all day and this could be a principal factor that drives its thermal behavior.
Surface salinity was lower (7.0 ± 0.7) in the 1998 sampling than in that of 2009 (14.5 ± 1.2) according to Peros et al. (2017). Intense precipitation events in Cayo Coco could have influenced this behavior in January and February of 1998. The total amount of rainfall in January (170.6 mm) is the second highest for this month according to the data from the Cayo Coco weather station. Total amount of rainfall of February 1998 (114.1 mm) also ranks second, slightly below the 128.1 mm of 1993 (historic record) for the said station. The sinkhole is located 2 Km away from the coast, which could prevent stronger saline intrusion. In the Yucatan Peninsula, salinity in most of the sinkholes surveyed was lower than in the Cenote Jennifer, including the ones near the coast (Elías Gutiérrez et al., 2007; Camargo-Guerra et al., 2013). However, some sinkholes like the Casa Cenote (located on the coast near Tulum, Mexico) is characterized by an active exchange with marine water and had evident salinity differences between the upper and lower layer (Sánchez et al., 2002). Echtinger (2000) found salinity differences of 20.0 between surface and bottom with clear halocline after 16 m at a Cuban coastal sinkhole. Only Peros et al. (2017) described a halocline (between 12 and 14 m), with salinity of 25.07 at the bottom (salinity of Cayo Coco marine waters ranges between 36.0 and 37.5). Groundwater in Cayo Coco is characterized by high salinities (between 10.0 and 20.0) at approximately 0.93 m deep (Hernández Valdés, 2011). So, salinity at the Cenote Jennifer depends mainly on rain volumes and on the weak tide influence near the bottom (Kovacs et al., 2017). For the zone in the Yucatan Peninsula that is 0–0.4 km away from the coast, Beddows (2004) documented that due to low hydraulic conductivity (restricted size of the conduits), the salinity gradient is steep (referred to as mixing zone; Beddows, 2004). Whereas, in the zone located >0.4–10 km away from the coast (and in areas of high conduit density), the gradient is less extreme and the halocline position was lower than predicted by the Ghyben-Herzberg principle (Beddows, 2004).
The increase of concentrations of some major ions from surface to bottom corroborated that the marine environment has some influence over the sinkhole, because ions (K+, Mg2+, Na+ and Cl–) are more abundant in seawater than in other naturals waters (Fagundo and González, 2005).
Dissolved oxygen concentration was very variable in Jennifer, highly depending on precipitation and supply of organic matter from the surrounding forest. It was significant that in the samplings of January and February of 1998 there was hypoxia at 12 m, while in the 2009 samplings, this condition occurred in the second sampling of April. In these three samplings, rain was typical of the dry season. Brankovits et al. (2017) found that rainfall was the key external factor regulating electron acceptor availability in the meteoric portion of one aquifer in the Yucatán Peninsula.
Peros et al. (2017) found lower DO concentration (1.8 mg l−1) than in our samplings, with a clear oxycline after depth of 5 m, but this sampling was in June of 2014 (wet season). During rainfall events, the organic matter that comes to the sinkhole is oxidized by aerobic heterotrophs that use oxygen (remineralization process), a normal behavior for lentic sinkholes (Schmitter-Soto et al., 2002; Cervantes-Martínez et al., 2002; Beddows et al., 2006, Ramos et al., 2017). Most of DO profiles (including of Peros et al., 2017) coincided that higher DO values are between 3 and 5 m, which could be related to higher photosynthetic production due to primary producers, with a high light-related variability. The Cenote Jennifer is surrounded by a dense forest, a light-limiting factor to primary producers (Díaz-Arce et al., 2001; Beddows et al., 2006). The significant correlation (negative) of DO with sulfide and CO2 (negative) is an expression of the biogeochemical process at the sinkhole. If dissolved oxygen is completely consumed by aerobic heterotrophs, alternate electron acceptors (e.g., sulfate from seawater or nitrate from meteoric groundwater) may be available for microbes to continue degrading organic matter trapped within the mixing zone (Ruberg et al., 2005; Pohlman, 2011; Brankovits et al., 2017).
In sinkholes with little human activity and with predominant supply of organic matter from the surrounding forest, nutrient concentrations were low, mainly because of rainfall volumes (Cervantes-Martínez et al., 2002; Schmitter-Soto et al., 2002; Elías Gutiérrez et al., 2007, Camargo-Guerra et al., 2013). Some authors attributed this behavior to high nutrient turnover rates (Cervantes-Martínez et al., 2002) and in the case of phosphorus, to the calcareous rocks that favor its co-precipitation with calcium, abundant in the karstic environment. The latter caused a P limitation in these systems, like in the Cenote Jennifer, where the N:P relation was greater than 10 in all the 2009 samplings.
Like in most sinkholes without high human impacts (Cervantes-Martínez et al., 2002; Schmitter-Soto et al., 2002; Elías Gutiérrez et al., 2007, Camargo-Guerra et al., 2013), the Cenote Jennifer is an oligotrophic system. Using the limits proposed by OECD (1982) for TP, the Cenote Jennifer can be classified as oligotrophic.
In this sinkhole, vertical variation of metabolic rates had mixed results, with greater values between surface and 7 m, coincident with the DO peaks and greater light availability. The rates of community metabolism and biogeochemical processes in the sinkhole are strong, depending on the amount of rainfall (by the supply of organic matter from surrounding forest) and light (Brankovits et al., 2017). These two factors could cause stratification due to the temporal halocline and oxycline presence. The net autotrophic character of the sinkhole is probably related to the amount of nutrients and organic matter received from the surrounding forest. At the bottom layer, anaerobic and aerobic (when mixing occurs) respiration prevail. In lentic sinkholes, this is a normal behavior and all physicochemical water conditions are strongly related to the dynamics of organic matter remineralization and the use of removed nutrients by some primary producers. However, the production of organic matter in situ is proportional to other factors, particularly light, scarce in sinkholes like Cenote Jennifer, surrounded by a dense forest (Beddows et al., 2006).
The low GPP recorded in Cenote Jennifer during the four samplings suggests that it is a low productive aquatic system, consistent with its oligotrophic conditions. We did not find any metabolism community experiment in the literature; most of them used chlorophyll a as an indicator of productivity. Some authors found high, medium and low productivity at some sinkholes in the Yucatan Peninsula (Díaz Arce et al., 2001; Cervantes-Martínez et al., 2002; Schmitter-Soto et al., 2002; Cervantes-Martínez et al., 2009; Camargo-Guerra et al., 2013; Cervantes-Martínez and Gutiérrez-Aguirre, 2015).
4.2 Management and conservation considerations
Sinkholes were very important to the indigenous peoples of the Caribbean islands and the Yucatan Peninsula, where they were used both, as sources of freshwater and also for ritualistic activities (Keegan and Carlson, 2008; Cooper, 2010). However, in Cayo Coco there are no archeological evidences of Cuba's aboriginal peoples. The Cenote Jennifer treasures rich paleo and biogeochemical records in its sediments. In this scenario, and taking into account the importance of paleoclimate studies to explain whether anthropogenic cause for Climate Change, we consider that this sinkhole must be preserved. The first step must be to keep it as tourist unexploited site and then, corroborate the existence of anchialine caves connected to the sinkhole. If so, cave diving could be a great option for specialized tourism as another product of the Jardines del Rey destination, particularly Cayo Coco.
Maximum density of use (MDU) calculated for Cenote Jennifer, taking into account 4 m2 of estimated vital area and a rest area of 30 m2, was of 107 visitors simultaneously. However, this sinkhole is not used as a swimming or bathing site in Cayo Coco, because Cayo Coco has beautiful sandy beaches. Many of the sinkholes in the Yucatan Peninsula, currently used for swimming and other tourist activities are over exploited and in dangerous conditions due to chemical, fecal and garbage contamination (Enseñat-Soberanis et al., 2019).
Illegal disposal of garbage is one of the major environmental problems in Cayo Coco and in the forest surrounding the Cenote Jennifer. This situation must be managed to avoid future contamination of this aquatic resource. Currently, there is no evidences of possible chemical contamination from infiltration through the aquifer of sewage treated in a nearby plant. Undoubtedly, this is good news for the sinkhole conservation strategies.
5 Summary
The Cenote Jennifer is an important and unique aquatic site in Cayo Coco (Jardines del Rey Tourist Destination) that has brackish to euhaline water, depending on the season and with some degree of communications with marine water. It is a lentic and oligotrophic system with a very low productivity and most of the time with anoxic waters at the bottom. However, it is of great scientific importance as evidence of paleoclimate records have been found in its sediments. The sinkhole is subject to some threats like garbage disposal in the surrounding forest and probable over exploitation for tourist purposes. At present, its conservation status is good and must be preserved as a scientific and specialized diving site in the future.
References
- Alcolado PM, Menéndez F, García-Parrado P, Zúñiga D, Martínez-Daranas B, Losa M, Gómez R. 1998. Cayo Coco, Sabana-Camagüey Archipelago, Cuba. In: Kjerfve N CARICOMP − Caribbean coral reef, seagrass and mangrove sites., editor Paris (France): UNESCO. p. 221–228. [Google Scholar]
- Alcolado PM, García EE, Arellano-Acosta M. 2007. Ecosistema Sabana-Camagüey: estado actual, avances y desafíos en la protección y uso sostenible de la biodiversidad. La Habana (Cuba): Editorial Academia. [Google Scholar]
- American Public Health Association, American Water Work Association, Water Pollution Control Federation (AHPA). 1985. Standard Methods for the Examination of Water and Waste Water, 16th edn. Washington, DC. 1268 pp. [Google Scholar]
- Batista Tamayo LM, González De Zayas R, Zúñiga Ríos A, Matos Pupo F, Hernández Roque L, González Alfonso D. 2006. Atributos físicos del norte de la provincia Ciego de Ávila. En: Ecosistemas costeros: biodiversidad y gestión de recursos naturales. Compilación por el XV Aniversario del CIEC. Sección I. Ecosistema del norte de la provincia Ciego de Ávila. CIEC. Editorial CUJAE. ISBN: 959-261-254-4. [Google Scholar]
- Beddows PA. 2004. Groundwater hydrology of a coastal conduit carbonate aquifer: Caribbean coast of the Yucatán Peninsula, México. Doctoral dissertation. University of Bristol. [Google Scholar]
- Beddows PA, Blanchon P, Escobar E, Torres O. 2006. Los cenotes de la Península de Yucatán. Arqueolog Mexicana 14: 32–35. [Google Scholar]
- Beddows PA, Smart PL, Whitaker FF, Smith SL. 2007. Decoupled fresh–saline groundwater circulation of a coastal carbonate aquifer: spatial patterns of temperature and specific electrical conductivity. J Hydrol 346: 18–32. [CrossRef] [Google Scholar]
- Brankovits D, Pohlman JW, Niemann H, Leigh MB, Leewis MC, Becker KW, Iliffe TM, Alvarez F, Lehmann MF, Phillips B. 2017. Methane- and dissolved organic carbon-fueled microbial loop supports a tropical subterranean estuary ecosystem. Nat Commun 18: 1835. [Google Scholar]
- Brinkmann R, Parise M, Dye D. 2008. Sinkhole distribution in a rapidly developing urban environment: Hillsborough County, Tampa Bay area, Florida. Eng Geol 99: 169–184. [Google Scholar]
- Camargo-Guerra T, Escalera-Vázquez LH, Zambrano L. 2013. Fish community structure dynamics in cenotes of the Biosphere Reserve of Sian Ka'an, Yucatán Peninsula, Mexico. Rev Mex Biodivers 84: 901–911. [CrossRef] [Google Scholar]
- Cervantes-Martínez A, Elías-Gutiérrez M, Suárez-Morales E. 2002. Limnological and morphometric data of eight karstic system “cenotes” of the Yucatan Peninsula, Mexico, during the dry season (February–May, 2001). Hydrobiologia 482: 167–177. [Google Scholar]
- Cervantes-Martínez A, Mezeta-Barrera M, Gutiérrez-Aguirre MA. 2009. Limnología básica del lago cárstico turístico Cenote Azul en Quintana Roo, México. Hidrobiológica 19: 177–180. [Google Scholar]
- Cervantes-Martínez A, Gutiérrez-Aguirre MA. 2015. Physicochemistry and zooplankton of two karstic sinkholes in the Yucatan Peninsula, Mexico. J Limnol 74: 382–393. [Google Scholar]
- Cervantes-Martínez A, Gutiérrez-Aguirre MA, Elías-Gutiérrez M, Arce-Ibarra AM, García-Morales A. 2018. Aquatic biodiversity in cenotes from the Yucatan Peninsula (Quintana Roo, Mexico). Teoría y Praxis 14: 49–68. [Google Scholar]
- Cooper J. 2010. Pre-Columbian archaeology of Cuba − a study of site distribution patterns and radiocarbon chronologies. In: Fitzpatrick SM, Ross AH, editors. Island Shores, Distant Pasts − Archaeological and Biological Approaches to the Pre-Columbian Settlement of the Caribbean. Gainesville (FL): University Press of Florida. p.81– 107. [Google Scholar]
- Díaz-Arce V, Herrera-Silveira JA, Comín FA. 2001. Limnological characteristics of two types of cenotes of Yucatán, Int Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 27: 3579–3582. [Google Scholar]
- Echtinger H. 2000. Die caverna Tanque Azul. Endins: publicació d'espeleologia 23: 145–154. [Google Scholar]
- Elías Gutiérrez M, Cervantes Martínez A, Gutiérrez Aguirre M, Arce Ibarra M. 2007. Los cenotes y lagunas del centro y sur de la península de Yucatán. En: Guadalupe De la Lanza Espino (Comp.) Las aguas interiores de México: conceptos y casos. p. 424–445. Editorial AGT. México. [Google Scholar]
- Emmert JA. 2016. Sedimentation in Submerged Sinkholes in Fillman's Creek, A Microtidal Estuary in Western Florida. Doctoral dissertation. Texas A&M University, USA. [Google Scholar]
- Enseñat-Soberanis F, Blanco-Gregory R, Mondragón-Mejía J, Simoes N, MorenO-Acevedo E, Ortega I. 2019. Crowding standards and willingness to pay at cenotes (sinkholes) of the Yucatan Peninsula: a comparative analysis of local, national and international visitors. J Ecotour 19: 1–22. [CrossRef] [Google Scholar]
- Fagundo J, González P. 2005. Hidrogeoquímica. La Habana, Cuba: Centro Nacional de Medicina Natural y Tradicional (CENAMENT). Ministerio de Salud Pública. [Google Scholar]
- Franco GL, Torre ADL. 1980. Los depósitos costeros del sur de la isla de la Juventud (Isla de Pinos), Cuba. Simposium XXXV Aniversario de la Sociedad Espeleológica de Cuba. Academia de Ciencias de Cuba, Isla de Pinos, 10 al 17 de agosto de 1975. [Google Scholar]
- García Debrás A, Pérez González A, Ortiz M. 1997. Distribución geográfica de los crustáceos peracáridos acuáticos (Mysidacea, Amphipoda, Isopoda) de las cuevas de Cuba. Cocuyo 6: 33–36. [Google Scholar]
- García Hernández M. 2001. Capacidad de acogida turística y gestión de flujos de visitantes en conjuntos monumentales: el caso de la Alhambra. PH: Boletín Del Instituto Andaluz Del Patrimonio Histórico 36: 124–137. [Google Scholar]
- García-Machado E, Hernández D, García-Debrás A, Chevalier-Monteagudo P, Metcalfe C, Bernatchez L, Casane D. 2011. Molecular phylogeny and phylogeography of the Cuban cave-fishes of the genus Lucifuga: evidence for cryptic allopatric diversity. Mol Phylogen Evol 61: 470–483. [CrossRef] [Google Scholar]
- Gazeau F, Borges AV, Barrón C, Duarte CM, Iversen N, Middelburg JJ, Delille B, Pizay MD, Frankignoulle M, Gattuso JP. 2005. Net ecosystem metabolism in a micro-tidal estuary (Randers Fjord, Denmark): evaluation of methods. Mar Ecol Progr Ser 301: 23–41. [CrossRef] [Google Scholar]
- G'meiner AA. 2016. Holocene environmental change inferred from fossil pollen and microcharcoal at Cenote Jennifer, Cayo Coco, Cuba. Mastership dissertation. McGill University. [Google Scholar]
- Gonzalez BC, Iliffe TM, Macalady JL, Schaperdoth I, Kakuk B. 2011. Microbial hotspots in anchialine blue holes: initial discoveries from the Bahamas. Hydrobiologia 677: 149–156. [Google Scholar]
- Guarch Rodríguez JJ, Corella Varona JE. 2010. Contribución a la geoespeleología de la mayor Caverna inundada de Cuba: Tanque Azul, Gibara, Holguín. Espelunca 9: 1–8. [Google Scholar]
- Gutiérrez-Domech R. 1998. El Karst en el archipiélago cubano y la región Caribe–Antillana. Minería y Geología 15: 39–52. [Google Scholar]
- Hall FG. 1936. Physical and chemical survey of cenotes of Yucatan. In: Pearse AS, Creaser AP and Hall FG. (eds.). The cenotes of Yucatán. Washington, DC: Carnegie Institute of Washington, pp. 5–16. [Google Scholar]
- Hernández Valdés AO. 2011. Selección de obras de captación y políticas de explotación en acuíferos costeros. Rev Ingen Hidráulica Ambiental 32: 3–9. [Google Scholar]
- Herrera-Silveira J, Comín F. 2000. An introductory account of the types of aquatic ecosystems of Yucatan Peninsula (SE México). In: Munawar M, Lawrence SG, Munawar IF and Malley DF. (Eds.), Aquatic Ecosystems of Mexico: Status and Scope. Leiden: Backhuys, pp. 213– 227. [Google Scholar]
- Holthuis LB. 1974. Subterranean Crustacea Decapoda Macrura collected by Mr. L. Botosaneanu during the 1973 Cuban-Roumanian Biospeological Expedition to Cuba. Int J Speleol 6: 231–242. [CrossRef] [Google Scholar]
- Humphreys WF. 1999. Physico-chemical profile and energy fixation in Bundera Sinkhole, an anchialine remiped habitat in north-western Australia. J Roy Soc Western Austr 82: 89–98. [Google Scholar]
- Humphreys WF, Poole A, Eberhard SM, Warren D. 1999. Effects of research diving on the physico-chemical profile of Bundera Sinkhole, an anchialine remiped habitat at Cape Range, Western Australia. J Roy Soc Western Australia 82: 99–108. [Google Scholar]
- Iliffe TM, Kornicker LS. 2009. Worldwide diving discoveries of living fossil animals from the depths of anchialine and marine caves. Smithsonian Contrib Mar Sci 38: 270–280. [Google Scholar]
- Iturralde‐Vinent MA. 1994. Cuban geology: a new plate‐tectonic synthesis. J Petrol Geol 17: 39–69. [CrossRef] [Google Scholar]
- Keegan WF, Carlson LA. 2008. Talking Taino: Caribbean Natural History from a Native Perspective. Tuscaloosa (AL): The University of Alabama Press. [Google Scholar]
- Keeton VK. 2017. The Control of Both Climate and Holocene Sea-Level Rise on Aquatic Environmental Conditions in a Coastal Sinkhole on Andros Island, the Bahamas. Doctoral dissertation. Texas A&M University, USA. [Google Scholar]
- Kovacs SE, Reinhardt EG, Stastna M, Coutino A, Werner C, Collins SV, Devos F, Le Maillot C. 2017. Hurricane Ingrid and Tropical Storm Hanna's effects on the salinity of the coastal aquifer, Quintana Roo, Mexico. J Hydrol 551: 703–714. [CrossRef] [Google Scholar]
- Mulec J, Oarga A. 2014. Ecological evaluation of air and water habitats in the Great Cavern of Santo Tomás, Cuba. Rev Mexicana Biodiversidad 85: 910–917. [CrossRef] [Google Scholar]
- Navarro-Mendoza M. 1988. Inventario íctico y estudios ecológicos preliminares en los cuerpos de agua continentales en la reserva de la biósfera de Sian Ka'an y áreas circunvecinas en Quintana Roo, México. (Informe técnico). CIQRO/CONACYT/USFWS, Chetumal. [Google Scholar]
- Núñez Jiménez A, Panos V, Stelcl O. 1968. Carsos de Cuba. Editora Nacional de Cuba. [Google Scholar]
- Organización para la Cooperación y el Desarrollo Económico (OECD). 1982. Eutrophication of Waters. Monitoring, Assessment and Control. Cooperative Programmers on Monitoring of Inland Waters (Eutrophication Control), Environment Directorate, OECD Paris, Final Report. France. [Google Scholar]
- Oficina Nacional de Estadística e Información (ONEI). 2018. Anuario Estadístico de Cuba 2017. Editorial Oficina Nacional de Estadística. [Google Scholar]
- Pérez-García JA, Díaz-Delgado Y, García-Machado E, Martínez-García A, González BC, Worsaae K, Armenteros M. 2018. Nematode diversity of freshwater and anchialine caves of Western Cuba. Proc Biol Soc Washington 131: 144–155. [CrossRef] [Google Scholar]
- Peros M, Collins S, G'meiner AA, Reinhardt E, Matos Pupo F. 2017. Multistage 8.2 kyr event revealed through high‐resolution XRF core scanning of Cuban sinkhole sediments. Geophys Res Lett 44: 7374–7381. [Google Scholar]
- Pohlman JW. 2011. The biogeochemistry of anchialine caves: progress and possibilities. Hydrobiologia 677: 33–51. [Google Scholar]
- Ramos J, Gracia J, Ortiz S, González-Chávez JL, Iturbe R. 2017. Water quality in aguadas within a protected karstic rain forest: the role of the vegetation-soil-water interactions. Ecol Eng 121: 2–11. [Google Scholar]
- Reynaldo E, Antonio A, Fernández A, Córdova E. 2016. Distribución y similitud de los peces dulceacuícolas del municipio Gibara, Holguín, Cuba. Novit Caribaea 10: 71–86. [CrossRef] [Google Scholar]
- Ruberg SA, Coleman DF, Johengen TH, Meadows GA, Van Sumeren HW, Lang GA, Biddanda BA. 2005. Groundwater plume mapping in a submerged sinkhole in Lake Huron. Mar Technol Soc J 39: 65–69. [Google Scholar]
- Salomon JN. 2019. Relations entre karsts, aquifères et niveaux de la mer à Cuba. Hommes et Terres du Nord 1995/1-2: 82–96. [Google Scholar]
- Sánchez M, Alcocer J, Escobar E, Lugo A. 2002. Phytoplankton of cenotes and anchialine caves along a distance gradient from the northeastern coast of Quintana Roo, Yucatan Peninsula. Hydrobiologia 467: 79–89. [Google Scholar]
- Sánchez y Pinto IA, Cervantes-Martínez A, Herrera RAG, Campos MEV, Gutiérrez-Aguirre MA. 2015. Evidencia de flujo preferencial al mar, del Cenote Caletita, en Cozumel, México. Ingeniería 19: 1–12. [Google Scholar]
- Schmitter-Soto JJ, Comín FA, Escobar-Briones E, Herrera-Silveira J, Alcocer J, Suárez-Morales E, Elías-Gutiérrez M, Díaz-Arce V, Marín E, Steinich B. 2002. Hydrogeochemical and biological characteristics of cenotes in the Yucatan Peninsula (SE Mexico). Hydrobiologia 467: 215–228. [Google Scholar]
- Silva Taboada G. 1974. Fossil Chiroptera from cave deposits in Central Cuba with description of two new species (genera Pteronotus and Mormoops) and the first West Indian record of Mormoops megalophylla . Acta Zool cracoviensia 9: 33–73. [Google Scholar]
- Somaratne N. 2017. Karst conduit networks, connectivity and recharge dynamics of a sinkhole. Environ Nat Resour Res 7: 70–88. [Google Scholar]
- Suárez Morales E, Rivera Arriaga E. 1998. Zooplacton e hidrodinámica en zonas litorales y arrecifales de Quintana Roo, México. Hidrobiológica 8: 19–32. [Google Scholar]
- Tamalavage AE, Van Hengstum PJ, Louchouarn P, Molodtsov S, Kaiser K, Donnelly JP, Albury AN, Fall PL. 2018. Organic matter sources and lateral sedimentation in a Bahamian karst basin (sinkhole) over the late Holocene: Influence of local vegetation and climate. Palaeogeogr Palaeoclimatol Palaeoecol 506: 70–83. [Google Scholar]
- UNESCO. 1983. Algorithms for computation of fundamental properties of seawater . SCOR/ICES/IAPSO Joint Panel on Oceanographic Tables and Standards and SCOR Working Group 51. [Google Scholar]
- Valdespino-Castillo PM, Merino-Ibarra M, Jiménez-Contreras J, Castillo-Sandoval SF, Ramírez-Zierold JA. 2014. Community metabolism in a deep (stratified) tropical reservoir during a period of high water-level fluctuations. Environ Monitor Assess 186: 6505–6520. [CrossRef] [Google Scholar]
- Wetzel RG, Likens GE. 1991. Limnological analyses. New York: Springer-Verlag, 391 p. [Google Scholar]
- Wetzel RG. 2001. Limnology. Lake and River Ecosystems, 3rd edn. San Diego: Academic Press, 1006 p. [Google Scholar]
- Young C, Martin JB, Branyon J, Pain A, Valle‐Levinson A, Mariño‐Tapia I, Vieyra MR. 2018. Effects of short‐term variations in sea level on dissolved oxygen in a coastal karst aquifer, Quintana Roo, Mexico. Limnol Oceanogr 63: 352–362. [Google Scholar]
Cite this article as: González-De Zayas R, de León LLP, Rodríguez LG, Pupo FM, Hernández-Fernández L. 2020. Limnological characteristics, community metabolism and management strategies of a coastal sinkhole in Cuba (Cenote Jennifer). Ann. Limnol. - Int. J. Lim. 56: 24
All Tables
Mean values of each measured parameter per depth in Cenote Jennifer in 1998 and 2009. Minor bold letters denote marked correlation among parameters. Major letters denote significant differences (p < 0.05) only in surface samples among samplings.
All Figures
 |
Fig. 1 Location figures and partial view of Cenote Jennifer, Cayo Coco, Cuba. |
| In the text | |
 |
Fig. 2 PCA analysis for all measured parameters in Cenote Jennifer in 1998. |
| In the text | |
 |
Fig. 3 Vertical profiles of (A) temperature (°C), (B) salinity, (C) pH and (D) dissolved oxygen (mg/L) in cenote Jennifer in 1998 samplings. |
| In the text | |
 |
Fig. 4 Vertical profile of temperature (°C) in cenote Jennifer in 2009 samplings. |
| In the text | |
 |
Fig. 5 Vertical profiles of (A) GPP, (B) NPP and (C) R (in mg O2 m−3 h−1) in Cenote Jennifer in 2009 experiments. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


