| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 56, 2020
|
|
|---|---|---|
| Article Number | 20 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/limn/2020018 | |
| Published online | 05 August 2020 | |
Research Article
Effects of salinity on species composition of zooplankton on Hau River, Mekong Delta, Vietnam
1
Can Tho University and staff of Faculty of Agriculture and Food Science, Tien Giang University, Can Tho, Vietnam
2
GRECO, Institute of Aquatic Ecology, Faculty of Sciences, University of Girona, Girona, Spain
3
College of Aquaculture and Fisheries, Can Tho University, Can Tho, Vietnam
* Corresponding author: vnut@ctu.edu.vn
Received:
12
March
2020
Accepted:
15
July
2020
The area surrounding the Hau River is one of the most important aquaculture and fisheries areas in the Mekong Delta, Vietnam. Fish, shrimp farms and fishers rely of the natural zooplankton production in the incoming water to sustain production. Zooplankton samples were collected from July 2017 to June 2018 using a zooplankton net with mesh size of 60 μm at 3 sites on Hau river at Tran De (river mouth), Dai Ngai (midpoint) and Cai Con (farthest salt intrusion area on Hau river). Qualitative and quantitative samples of zooplankton together with salinity level were determined monthly at each sites. The salinity was found to fluctuate from 0 to 20‰ in the study area. A total of 137 zooplankton species were recorded including 26 species of Protozoa (19%), 47 species of Rotifera (34%), 12 species of Cladocera (9%), 44 species of Copepoda (32%) and 8 other taxon (6%). Copepod and rotifer prevailed with high densities (19.9 × 103 ind m−3 and 19.7 × 103 ind m−3, respectively), whereas protozoa and cladocera were less abundant with 6.8 × 103 ind m−3 and 4.9 × 103 ind m−3, respectively. When salinity increased to more than 5, protozoa and copepods were more abundant and reached a peak at 20 with 25.0 × 1036 ind m−3 and 53.0 × 103 ind m−3, respectively. Regression analysis indicated that the density of zooplankton was significantly correlated to salinity variation. Protozoa and copepod were positively correlated with salinity, whereas cladocera and rotifer were negatively correlated with salinity. The impacts of climate change could exacerbate the seasonal fluctuations in salinity and zooplankton composition.
Key words: Hau River / Mekong Delta / salinity changes / zooplankton composition / zooplankton structure
© EDP Sciences, 2020
1 Introduction
Zooplankton communities are of the first important links of the food webs in aquatic ecosystems. Their functions also help balance the ecosystem, maintain and enhance biological productivity of a water body. In aquaculture, zooplankton are the primary food source with high nutritional value, which are indispensable during the larval rearing period of aquatic animals (Lavens and Sorgeloos, 1996; Das et al., 2012) and in aquaculture ponds (Porchas-Cornejo et al., 2013; Anton-Pardo and Adámek, 2015). Zooplankton, however are very sensitive to environmental changes. Any factor impacting on water body will affect the structure and abundance of zooplankton. Therefore changes in zooplankton structure or composition can be an indicator of changes of the environment. Many studies have showed that saline intrusion and salinity are important factors controlling species composition and biomass of zooplankton communities in coastal estuarine ecology (Valdes and Moral, 1998; Nielsen et al., 2003; Anton-Pardo and Armengol, 2011; Steinberg et al., 2015). Changes in zooplankton structure may result in changes in food webs which could affect the productivity of the ecosystems. Importance roles of zooplankton on fish and fisheries production both in natural and farmed conditions have been noticed by a quite number of researches. Fernando (1994) emphasized the importance of zooplankton to fish yields and fisheries in the tropic freshwater areas. Ludwig (1999) described the succession of zooplankton in a freshwater pond and stressed the improtance of rotifers as initially small prey of the young fish. Recently, Rajashree et al. (2017) mentioned the structure and important role of zooplankton in the rice fields as the natural food sources, especially cladocerans for fishes concurrently in this habitat. Amian (2018) also confirmed the indispensable role of zooplankton in which rotifers play as food source for fish larvae in the extensive fish ponds where they present with highest richness and abundance. In shrimp ponds, zooplankton were determined as the crucial natural food for the postlarvae immediately after stocking (Coman et al., 2003; Anton-Pardo and Adámek, 2015).
Hau River is one of the two large tributaries of the Mekong River. It provides important ecosystem services to local people and it is crucial for the development of aquaculture in the Mekong Delta (Quyen and Amararatne, 2016). In recent years, saline intrusion has increased in the Mekong Delta in general and in Hau River basin in particularly. Severed intrusion of salinity in the Mekong Delta due to climate change, sea level rise and reduction of freshwater from the upstream in the dry season was projected by many studies (Hoanh et al., 2003; Tuan et al., 2007; Nhan et al., 2007; Sunada, 2009). Recent report has confirmed the serious salt intrusion thriven not only by the above mentioned causes but also by other anthropogenic activities that exacerbate the circumstances in the Mekong Delta (Eslami et al., 2019). It has caused substantial damage to agriculture and aquaculture in the region (Duyen et al., 2012; Tri, 2016). Salinization may have a strong impact on the fisheries productivity as it changes the structure of zooplankton community and food webs. Productivity of freshwater fish farming systems such as rice-fish, fish pond culture along Hau River may be impacted. With a similar trend, fisheries production on this important river may also be affected. Little is known about the zooplankton composition on Hau River, especially in the estuary (Cho et al., 2012; Lien et al., 2014). Moreover, these studies focused mainly on species diversity and composition rather than structure variation under salinity changes. The aim of this study was therefore to provide data of changes in plankon in general and zooplankton in specific under salinity alterations especially severed salt intrusion by climate change impact in the Mekong Delta, Vietnam. Changes of zooplankton composition under salinity changes can reflect changes in zooplankton composition and food web structure and ultimately fish productivity. This discovery would for the first time provide important database for prediction of productivity due to zooplankton composition and abundance changes in the surrounding areas of Hau River, especially areas affected by salinity intrusion in the Mekong Delta.
2 Methods
2.1 Time and site of study
The study was conducted from July, 2017 to June, 2018 in the lower reaches of the Hau River belonging to Soc Trang province. As mentioned earlier, Hau River is the largest tributary of Mekong River and running through 4 provinces in the Vietnamese territory including An Giang (upper reach), Can Tho and Hau Giang (middle reach) and Soc Trang (lower reach) provinces which are parts of the Mekong Delta. The weather in the Mekong Delta is characterized by two distinct seasons, a rainy season (from May to October) and a dry season (from November to April). In the study area, zooplankton samples were collected monthly throughout the two seasons at 3 sites on Hau River: (I) Cai Con (9°55′48.9″- 105°54′02.6″) is the most upstream point of the river (about 60 km from the river mouth) that is reached by the saline intrusion that occurs during the dry season); (II) Dai Ngai (9°43′47.2″–106°04′52.4″) the middle site; and (III) Tran De river mouth (9°28′0.90″ − 106°14′35.5″) (Fig. 1).
 |
Fig. 1 Hau River and 3 sites (I, II, III) for zooplankton sampling. |
2.2 Sample collection
Quantitative and qualitative zooplankton samples were collected at a depth of 30 cm at both at high tide and low tide as Hau River is featured with semi-tidal regime. The quantitative samples were taken by using a 20 L bucket to scoop water from different points within the sampling site and filtering through a 60 μm mesh size zooplankton net with a total volume of 400 L. The qualitative samples were collected by dragging the net along the sampling site for about 5 minutes. Both samples were preserved in 110 mL plastic bottles with formalin (38%) at 4-6% and transported to be analysed at the laboratories of College of Aquaculture and Fisheries, Can Tho University. With each sample, salinity was also recorded using a refractometer at all sites and periods.
2.3 Sample analysis
Qualitative samples were analysed by identifying all zooplankton species using the published taxonomy keys including Shirota (1966), Chen (1980) and Dang et al. (2015). Densities (ind m−3) of zooplankton were determined on the species basis using Sedgewick-Rafter and Bogorov counting chambers. Before counting the samples were screened through a 200 μm mesh size net. Large specimens were counted thoroughly by the Bogorov chamber and the rest was counted by the Sedgewick-Rafter chamber. Densities of each species of the four main zooplankton groups (Protozoa, Rotifera, Cladocera and Copepoda) were determined based on the following formula for the Sedgewick-Rafter chamber: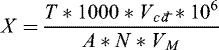 where X density of zooplankton species (ind m−3); T: number of individuals of each species counted, A: area of a counting square (1 mm2); N: number of counting squares = 180; Vcđ
: sample concentrated volume (mL); VM
: sampling sample volume in the field.
where X density of zooplankton species (ind m−3); T: number of individuals of each species counted, A: area of a counting square (1 mm2); N: number of counting squares = 180; Vcđ
: sample concentrated volume (mL); VM
: sampling sample volume in the field.
Those counted by Bogorov chamber were determined with the following formula: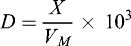 where D: density of zooplankton species (ind m−3); X: number of individuals of each species counted; VM
: sampling sample volume in the field.
where D: density of zooplankton species (ind m−3); X: number of individuals of each species counted; VM
: sampling sample volume in the field.
Total density of a species or group was a sum determined from the above two formulas.
Bio-indices including Margalef species richness (d), Shannon diversity index (H ′), Simpson dominant species (λ) and Pielou similarity index (J ′) were applied to assess the structure of zooplankton community.
2.4 Data analysis
Data were analysed using different specialized software including SPSS 16.0, Primer 7.0.13, R 3.6 and R. studio. In order to fully assess the impact of salinity on the structure of the zooplankton community which is composed of only 4 main groups including Protozoa, Rotifera, Cladocera and Copepoda, analysis and assessment were carried out in two ways. Firstly, (i) the zooplankton composition was assessed at different salinity ranges based on species number (SN), percentage of appearance (%) of species which is the proportion of each zooplankton group to total species of zooplankton, population density (ind m−3) of each group of zooplankton. Secondly, (ii) the distribution of species and community density of zooplankton was assessed at different salinity using correlation and regression analysis. The structure of zooplankton community at different salinities was analysed by PCA correlation and Discriminant function analysis. PCA analysis was used to assess the similarity of distribution of zooplankton species under the effect of different salinities. The purpose of Discriminant function analysis is to determine the relationship between salinity and density of 4 main zooplankton groups (not for other taxa). Positive or negative correlation of salinity to 4 zooplankton group densities (Protozoa, Rotifera, Cladocera and Copepoda) is expected. Densities (ind m−3) of 4 zooplankton groups including Prorozoa (X1), Rotifera (X2), Cladocera (X3) and Copepoda (X4) are dependent factors and affected by fluctuation of salinity. Salinity range is the independent factor. Discriminant function analysis was started by computing the data of 4 plankton group densities as the independent variables (internal independence between 4 groups of zooplankton) and salinity data as the grouping variables. This analysis was completed by Fisher's test with a within group correlation to predict group of membership. From the results of Discriminant function analysis, an equation (Z) was built to predict the fluctuation of total zooplankton density based on 4 zooplankton groups which had been affected by salinity. The result of the output “Standardized Canonical Discriminant Function Coefficients” was used to calculate the equation (Z). In addition, the “Percent of variance of Eigenvalues” was used to explain the predictive power of the equation (Z). The predicted model result of total zooplankton density (ind m−3) is expressed by equation (Z) as follow:

where Z: total zooplankton density (ind m−3). Z is the predicted value when X1, X2, X3, X4 are fluctuated by salinities. X 1, X 2, X 3 and X 4: density (ind m−3) of Protozoa, Rotifera, Cladocera and Copepoda, respectively. α 1, α 2, α 3 and α 4: coefficients of Protozoa, Rotifera, Cladocera and Copepoda, respectively which were outputted from the Discriminant function analysis.
3 Results
3.1 Fluctuation of salinity
Throughout the year, salinity recorded at 3 different sampling sites on Hau River revealed a strong seasonal fluctuation ( Fig. 2).
From Figure 2, the actual salinity recorded at all sampling sites varied from 0 to 20. Salinity at Tran De was higher than that in Dai Ngai and salinity in Dai Ngai was higher than that in Cai Con. Salinity at high tide was higher than that at low tide.
In the rainy season which is from August to October, salinity was 0 at all sites and at both high and low tide (Fig. 2). However, in the early dry season in November salt intrusion started with 2 salinity detected at Tran De (river mouth). Increases in salinity were observed further landward from January onward. Strong saline intrusion occurred in the dry season from December to April. Highest salinity was recorded in March with 6 at Dai Ngai (ii) and 20 at Tran De (iii). At Cai Con, the farthest site from river mouth salinity reached a maximum of 1 at low tide and 2 at high tide in January is considered the most upstream point in the Hau River reached by the salinity intrusion. Salinity dropped sharply to 0 at three sampling sites at the beginning of the rainy season in June.
 |
Fig. 2 Fluctuation of salinity on Hau River throughout sampling periods. |
3.2 Composition of zooplankton on Hau River
3.2.1 Species composition and abundance
A total of 137 zooplankton species was recorded on Hau River in which 128 of them are holoplankton (see Appendix) including Rotifera accounting for highest number with 47 species (34%), followed by Copepoda with 44 species (32%), Protozoa, 26 species (19%) and Cladocera, 12 species (9%). In addition to the 4 main zooplankton groups, 8 other taxon (6%) were also noted as meroplankton including mollusc larvae, aquatic insects, Polychaeta, Nematoda and Chaetonagtha. Copepods and rotifers were the most abundant groups with the highest densities (19.9 × 103 ind m−3 and 19.7 × 103 ind m−3, respectively), followed by protozoans (6.8 × 103 ind m−3) and cladocerans (4.9 × 103 ind m−3) ( Fig. 3).
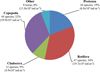 |
Fig. 3 Species composition and density (figures in the bracket) of zooplankton on Hau River. |
3.2.2 Diversity and bio-indices
Figure 4 shows a difference in Cumulative Dominance and Species rank between cladocera and other groups (protozoa, rotifer and copepod) on the Hau River. Cumulative Dominance increased fastest with Species Rank for the cladocera, with relative increase more similar for other 3 groups. This relationship indicates that the impact of salinity by tide and by time has a different effect on the relationship between Cumulative Dominance and Species rank of zooplankton community.
Diversity of zooplankton community on Hau River was assessed through different biodiversity indices including d, J′, H′, λ and presented in Table 1.
The results from biodiversity index analysis show that the Magalef species richness (d) of Protozoa, Rotifera, Cladocera and Copepoda was always >5.0 and ranged from 5.0 to 5.85 (Tab. 1). This indicates that zooplankton composition on Hau River was quite diverse. Species richness of cladocera was highest among 4 zooplankton groups with a value of 5.85 indicating that Hau River is a typical freshwater ecosystem. Similar to d, the Shannon diversity index (H ′) of 4 zooplankton groups was quite high ranging from 3.59 to 4.02. Highest H ′ was found for copepod, up to 4.03 (Tab. 1) indicating that copepod was a dominant zooplankton group in the river. In contrast, the H ′ of cladocera was lowest indicating that cladocera is less diverse compared to the other groups. The Pieloud similarity index (J ′) presented in Table 1 shows that the J ′index of zooplankton was in a range of 0.867 to 0.942. Highest J ′ index was found for copepod, reaching a peak of 0.942. This indicates that copepod has a highest similarity in the community. The similarity of cladocera was lowest with 0.867 (Tab. 1). Population structures of protozoa and rotifer were quite high in similarity as their H ′ index were high (0.940 and 0.917, respectively). The Simpson dominant species indices (λ) of zooplankton were quite low ranging from 0.20 to 0.43. It is clearly seen that copepod got a highest diversity among 4 groups of zooplankton and therefore obtained lowest λ of 0.020. In contrast, cladocera presented with lowest J′ leading to a highest λ of 0.34. This proved that dominant species richness in cladocera population was lowest. The dominant species richness of protozoa and rotifer populations were relatively high with λ index of 0.021 and 0.024, respectively (Tab.1).
 |
Fig. 4 Cumulative Dominance (%) and Species rank of 4 zooplankton groups on Hau River. |
Bio-indices of zooplankton on the Hau River.
3.3 Zooplankton with salinity changes
3.3.1 Species composition structure
As salinity changed gradually with a range of 0 to 20, effects of salinity on zooplankton species structure were assessed. At different salinities, the species composition of zooplankton on Hau River was remarkably different indicating that the fluctuation of salinity resulted in a strong effect on zooplankton species composition (species number and occurrence percentage) on Hau River ( Fig. 5). The number of rotifer and cladocera species varied with changes in salinity and tended to decrease with increasing salinity (0–20). At 0, rotifers were the most abundant group with 12 species (Fig. 5a) accounting for 49% of total zooplankton at all sampling sites. When salinity increased to 20 at the river mouth (Tran De) at high tide in March (Fig. 2), only one species of rotifer was recorded accounting for 5%. Cladocera was also found in most of the sites at 0 but a lower number of 3 species and only 12% by composition (Fig. 5a and 5b). However, when salinity increased to 10 at Tran De during low tide in January (Fig. 2), no species of cladocera was recorded.
Species number of protozoa and copepod also varied sharply with salinity changes. However, the fluctuation of these two groups was completely opposite to that of rotifer and cladocera as the number of species increased with increased salinity. Three species of protozoa were recorded at 3 in Dai Ngai (midpoint) at low tide in February to March and increased to 9 species at 20 in Tran De at high tide in March. Similarly, only 6 species of copepod were found at 0 but increased to 11 at 4, 10 at 11 and 10 species at 20 (Fig. 5).
The species structure of zooplankton on the Hau River was influenced by the changes in salinity as salt water intruded up the river. In freshwater rotifers were the most abundant group (49%). However, when salinity increased to the number of species and abundance of rotifers and cladocerans decreased. In contrast both species numbers and the species composition of copepods and protozoans increased with salinity (Fig. 5a,b).
 |
Fig. 5 Number of zooplankton species (5a), and % of appearance (5b) at different salinities |
3.3.2 Abundance
Similar to species composition structure, effects of salinity on abundance of zooplankton was also noticed ( Fig. 6). Increased densities of protozoans and copepods were recorded as salinities increased from 0 to 20. However, the opposite was observed for rotifers and cladocerans. Protozoa densities ranged 3.4 × 103–25.0 × 103 ind m−3 in which lowest density (3.4 × 103 ind m−3) was found at 3‰ in Dai Ngai at low tide from February to March (Fig. 2) and highest density (25.0 × 103 ind m−3) recorded in Tran De at high tide in March when salinity reached 20. Rotifers were found more abundant (23.3 × 103 ind m−3) at 0 than at 20 (6.3 × 103 ind m−3). Density of cladocera was highest (6.2 × 103 ind m−3) at 5 in Dai Ngai at high tide in April and reduced significantly at higher salinity (10) and were not present in the samples at salinities greater than 15. Copepods were the most abundant group in the river. When salinity was greater than 4, the density of copepod increased significantly and reached a peak of 53.0 × 103 ind m−3 at 20 at Tran De in March. However, when salinity decreased to less than 3, a substantial reduction in density of copepod was observed at 1 (12.1 × 103 ind m−3) in Tran De at low tide in July.
 |
Fig. 6 Fluctuation of zooplankton densities at different salinities. |
3.4 Correlation between salinity and distribution of zooplankton
3.4.1 Similarity in distribution at different salinities
Twenty one zooplankton species with high occurrence percentage and density (including 2 of protozoa, 10 of rotifer, 3 of cladocera and 6 of copepod) were selected for PCA analysis to assess the correlation between salinity and distribution of zooplankton.
The PCA results indicate that salinity was significantly correlated with distribution of zooplankton ( Fig. 7) and when added together PC1 and PC2 explained 62.5% of all the variability in the data. Salinity was the main factor influencing the distribution of rotifer species (Brachionus plicatilis Muller, 1786, Keratella tropica Apstein, 1907 and Keratella cochlearis Gosse, 1851), copepods (Paracalanus parvus Claus, 1863, Cyclops strenuus Fischer, 1851, Mesocyclops leuckarti Claus, 1857, Eucyclops serrulatus Fischer, 1851), protozoans (Tintinnopsis gracilis Kofoid and Campbell, 1929) and cladoceans (Moina brachiata Jurine, 1820, Ceriodaphnia megalops Sars, 1890). In addition, B. plicatilis (rotifer) and T. gracilis (protozoa) were found to have similar distribution and broad expansion with sites and times at low salinities of 0–4. P. parvus and Chiridiella macrodactyla Sars, 1907 (calanoid copepods) were strongly associated with higher salinities (10–20). In contrast, Eucyclops serrulatus Fischer, 1851, Mesocyclops leuckarti Claus, 1857 (cyclopoid copepods) and K. tropica (rotifer) were more associated with lower salinity, especially 0. Similarly, C. strenuus (copepod), M. brachiata, C. megalops (cladocera), Keratella valga Ehrenberg, 1834 (rotifer), Arcella polypora Penard, 1890 (protozoa) were also associated with low salinity (0–2).
 |
Fig. 7 PCA biplot of the Hellinger-transformed 21 zooplankton species under different salinities on Hau River (PC1 explained 24.8% var., PC2 explained 37.7% var.). |
3.4.2 Regression model of zooplankton density under salinity changes
The Discriminant function analysis result showed that when salinity fluctuated from 0–20, density of Protozoa and Copepoda had a positive correlation with salinity whereas density of Rotifera and Cladocera was negatively correlated with salinity. Hence, total density (ind m−3) of zooplankton was predicted by the following equation:
 (1)(P < 0.001, % of variance = 83.0%).
(1)(P < 0.001, % of variance = 83.0%).
As salinity rises zooplankton abundance increases, Equation (1) indicated that this increase is driven by copepods and protozoans with corresponding declines in the numbers of rotifers and cladoceans.
4 Discussion
4.1 Fluctuation of salinity on Hau River
Salinity on the Hau river was found to vary seasonally becoming freshwater even in the river mouth during the rainy season in August and October. Highest salinities were noted in the dry season, where small increases in salinity were even noticed at the upper most sampling point (Cai Con) and more strongly at the river mouth (Tran De). Similar trend was also found by Binh et al. (2018) who concluded that salinity is regularly diminished upstream-ward on Hau River due to the dilution of freshwater flowing down from the upstream. In Tien River (another branch of Mekong River in MD), Hung et al. (2018) also reported that salinity of surface water decreased significantly from river mouth to upstream during July to October annually. However, salinity increases during the dry season from December to April as sea water intruded further landward. This increased salinity occurred stronger at high tide than low tide. This phenomenon was also observed by Binh et al. (2018) on Hau River where saline intrusion was recorded much further at high tide. Salinization is reported to be an increasing problem in the Mekong Delta (Dat et al., 2012; CCAFS-SEA, 2016; Anh et al., 2018) having significant impact on crop production, freshwater supplies and freshwater ecosystems. Reduction of flow caused by climate change and anthropogenic activities, especially upstream dam construction may exacerbate problem of saline intrusion in the Mekong Delta. Although water flow and hydrological regimes are dependent upon many factors and complicated to predict (Dat and Likitdecharote, 2010 and Dat et al., 2012), the flow can be described mathematically and modelled by using computer simulation (MRC, 2009) which is commonly useful tool to predict salt intrusion under flow reduction, sea level rise or climate changes (Dat et al., 2012; Long et al., 2016; Li et al., 2017).
4.2 Effects of salinity on composition structure of zooplankton on Hau River
Ecologically, salinization or increased salinity would change the composition and densities of zooplankton (Zorina-Sakharova et al., 2014; Paturej and Gutkowska, 2015; Tavsanoglu et al., 2015). Gao et al. (2008) indicated that variation of salinity in the estuary resulting in regional and seasonal alteration of dominant species. This is true for the fact that zooplankton composition changed obviously from the river mouth to landward areas along Hau River with variable salinity. At low salinity range of 0–2, rotifers were most abundant with highest number of species. This record is consistent with results found by Zakaria et al. (2007) and Zorina-Sakharova et al. (2014) that in low-salinity water, rotifers are dominated in zooplankton communities. In addition, Bielanska-Grajner and Cudak (2014) also emphasized that in low-salinity water environments (less than 0.5), diversity of zooplankton depends on species of rotifers as low salinity or light-saline intrusion is the optimum conditions for rotifer populations to expand. Herzig (1987) also stated that rotifers were suitable for thriving in freshwater or low salinity. Another study in the estuary of north-eastern Brazil by Silva et al. (2009) also confirmed a rich species composition of rotifers recorded in the rainy season, when freshwater from inland areas flowed out and reduced salinity in the estuary. Similar to rotifers, cladocera was a zooplankton group that thrived at most of the sampling sites with low salinity (less than 5) on Hau River. This phenomenon was also recorded in the study of Spoljar et al. (2018) that cladocera was a typical group growing in freshwater or low salinity. When salinity in the estuary decreased due to rain, the species composition of cladocera became richer (Silva et al., 2009).
When saline intrusion occurred in the dry season, salinity increased to more than 7. The composition of zooplankton community changed remarkably from Dai Ngai to Tran De (from medium salinity to high salinity). Number of species and densities of protozoa and copepods consequently became richer whilst abundance (both species number and densities) of rotifers and clacocera decreased sharply. This explains positive and negative correlation to salinity of 2 distinct groups including protozoa and copepods, and rotifers and cladocera, respectively on Hau River. This trend was also described by Zakaria et al. (2007) and Spoljar et al. (2018) that rotifers and clacocera had a negative correlation with salinity while protozoa and copepods displayed a positive correlation with salinity. At 9, zooplankton composition structure was most stable and copepods were dominant with calanoids and harpactoids (Werba, 2016). In this study both calanoids and cyclopoids were found and the PCA analysis revealed clearly the distribution of these groups related to salinity. Paracalanus parvus and Chiridiella macrodactyla are calanoids and found at higher salinity (>10) while Eucyclops serrulatus, Mesocyclops leuckarti, Cylops strennus are cyclopoids and characterized with freshwater. Zakaria et al. (2007) also confirmed Paracalanus parvus grew strongly in high salinity water environments around more than 38.5. Other species of calanoids such as Arcatia tonsa Dana, 1849 was also recorded to well adapt to salinity from 15 to 20 (Cervetto et al., 1999). Abundance of copepods was also observed increasing with salinity increasing (Qian et al., 2008; Raybaud et al., 2008; Toruan, 2012; He, 2012; Prado et al., 2017). However, increasing of salinity lead to decrease or disappear of cladocera species (Anton-Pardo and Armengol, 2011). Another study had also emphasized that increased salinity would reduce species richness of cladocera, especially within 7–9 (Zorina-Sakharova et al., 2014). Similar to copepods, abundance of protozoa on Hau River was also positively increasing with increased salinity. When studying on the effects of salinity on structure of zooplankton community in Cu Lao Dung mangrove in Soc Trang province (in Mekong Delta), Lien et al. (2013) found that in the rainy season with low salinity (2–5), protozoa occurred with a proportion of 25% of zooplankton composition, but in the dry season when salinity decreased, the percentage of occurrence of protozoa increased to 50%.
In general, at higher salinity conditions, especially at Tran De estuary in the dry season, copepods were more abundant both in species number and density on Hau River. This finding is consistent to previous studies in different areas in the world as well as in Vietnam. For example copepods also increased with increasing salinity in Mondego estuary of Portugal (Vieira et al., 2003), in Aiguamolls de l'Emporda wetland of Spain (Balmana, 2004) and in the coastal area of the Mekong Delta, Vietnam from Soc Trang to Bac Lieu (Van et al., 2012).
Changing in zooplankton community in freshwater area leads to reduction of cultured fish production as the preferred preys (rotifers and cladocera) are replaced by unsuitable copepods or protozoa. Whereas, in the brackishwaters copepods are more preferably consumed by brackishwater fish or shrimp (Anton-Pardo and Adámek, 2015). Thus, there could be some advantages to marine or brackishwater fish species but more disadvantages to freshwater ones under salinization. Regardless of the direct impacts, an ecosystem must have been significantly altered ecologically as a whole. In the ecosystem, not only changes in zooplankton composition but more importantly in phytoplankton which are the food source of zooplankton. Changes in species composition of phytoplankton could negatively impact on the ecosystem and its surrounding environment as more dinoflagellates may invade with higher salinity into the estuaries. Dinoflagellates are also classified as protists (Ruppert and Barnes, 1994) more abundant in the coastal marine waters and many of them can produce toxins (Graham and Wilcox, 2000). Larsen and Nguyen (2004) described 70 species of potentially harmful microalgae from Vietnamese coastal waters including the Mekong estuary. Among these species, 75% are dinoflagellates. In addition to alteration of food web structure, effects of harmful dinoflagellates to aquaculture, especially shellfish culture are of much concern along the coastal areas (Matsuyama, 1999).
In conclusion, the structure of zooplankton species on lower reach of Hau River was typical of freshwater or oligohaline community in the rainy season. However, the composition structure changed with the saline intrusion during the dry season. As stronger saline intrusion are predicted to occur under impacts of upstream dam construction and climate change (less rain, drought, sea level rises) the zooplankton community might one day be characterised as brackish water. Only a small increase in salinity can cause significant upheavals in the zooplankton community as rotifers and cladocera were predominant in water of less than 5, while copepods dominated in all salinities of more than 5. These changes in zooplankton community could have significant impacts on Mekong Delta ecosystem including the fish populations in the Mekong Delta and the livelihoods of stakeholders.
Acknowledgements
This study was funded in part by the Can Tho University Improvement Project VN14-P6, supported by a Japanese ODA loan. The authors also thank Dr. Mark Walton, University of Wales Bangor, UK for his assistance with English language editing.
Appendix
List of 128 species belonging to four main zooplankton groups including Protozoa, Rotifera, Cladocera and Copepoda found in the estuarine areas of Hau River, Mekong Delta, Vietnam.
References
- Anh DT, Long HP, Duc BM, Rutschmann P. 2018. Simulating future flows and salinity intrusion using combined one- and two-dimensional hydrodynamic modelling − the case of Hau River, Vietnamese Mekong Delta. Water 10: 897p. [Google Scholar]
- Anton-Pardo M, Adámek Z. 2015. The role of zooplankton as food in carp pond farming: a review. J Appl Ichthyol 31 (Suppl. 2): 7–14. [Google Scholar]
- Anton-Pardo M, Armengol X. 2011. Effects of salinity and water temporality on zooplankton community in coastal Mediterranean ponds. Estuar Coast Shelf Sci 114: 93–99. [Google Scholar]
- Balmana SB. 2004. Zooplankton structure and dynamics in Mediterranean marshes (Emporda wetlands): a size-based approach. Doctoral dissertation. Institute of Ecological Aquatics, Faculty of Environmental Science, The University of Girona. [Google Scholar]
- Bielanska-Grajner I, Cudak A. 2014. Effects of salinity on species diversity of Rotifers in anthropogenic water bodies. Pol J Environ Stud 23: 27–34. [Google Scholar]
- Binh VD, Kantoush S, Sumi T, Nguyen PM, La VT. 2018. Field investigations on river bed changes and salinity intrusion along Tien and Hau rivers in Vietnamese Mekong Delta considering upstream dams' impacts. Disaster Prev Res Inst Annu 61(B): 770–783. [Google Scholar]
- Cervetto G, Gaudy R, Pagano M. 1999. Influence of salinity on the distribution of Acartia tonsa (Copepoda, Calanoida). J Exp Mar Biol Ecol 39: 33–45. [Google Scholar]
- CCAFS-SEA (CGIAR Research Program on Climate Change, Agriculture and Food Security- Southeast Asia). 2016. Assessment Report: The drought and salinity intrusion in the Mekong River Delta of Vietnam. Hanoi, Vietnam. [Google Scholar]
- Chen QC. 1980. The marine zooplankton of Hong Kong. The marine flora and fauna of Hong Kong and Southern China. Hong Kong: Hong Kong University Press. [Google Scholar]
- Cho N, Trinh TSH, Lam NN. 2012. Species diversity of zooplankton in coastal waters of Vietnam − family Acartidae (Copepoda). J Biol 34: 294–304. [Google Scholar]
- Coman FE, Connonly RM, Preston NP. 2003. Zooplankton and epibenthic fauna in shrimp ponds: factor influencing assemblage dynamics. Aquacult Res 34: 359–371. [CrossRef] [Google Scholar]
- Dang PD, Khoi NV, Nguyet LT, Thanh NDN, Hai TH. 2015. Identification handbook of freshwater zooplankton of the Mekong River and its tributaries, MRC Technical Paper 45. [Google Scholar]
- Das P, Mandal SC, Bhagabati SK, Akhtar MS, Singh SK. 2012. Important live food organisms and their role in aquaculture. Narendra Publishing House. [Google Scholar]
- Dat TQ, Likitdecharote K. 2010. Effect of Sea Level Rise and Low Flow on Salinity Intrusion in Mekong Delta. GMSTEC 2010: International Conference for a Sustainable Greater Mekong Subregion, 26–27 August 2010, Bangkok, Thailand. [Google Scholar]
- Dat TQ, Trung NH, Likitdecharote K. 2012. Saline intrusion simulation in the Mekong Delta under impacts of sea level rise and reduced upstream freshwater flow. J Sci Can Tho University 21b: 141–150. [Google Scholar]
- Duyen PLM, Tri VPD, Hieu NT. 2012. Assessing changes in land use systems under the impact of climate change and sea level rise in Vinh Chau district, Soc Trang province. J Sci Can Tho Univ 24a: 253–263. [Google Scholar]
- Eslami S, Hoekstra P, Nguyen Trung N, Ahmed Kantoush S, Van Binh D, Duc Dung D, van der Vegt M. 2019. Tidal amplification and salt intrusion in the Mekong Delta driven by anthropogenic sediment starvation. Sci Rep 9(1). doi:10.1038/s41598-019-55018-9. [Google Scholar]
- Fernando CH. 1994. Zooplankton, fish and fisheries in tropical freshwaters. Hydrobiologia 272: 105–123. [Google Scholar]
- Gao Q, Xu Z, Zhuang, P. 2008. The relation between distribution of zooplankton and salinity in the Changjiang Estuary. Chin J Ocean Limnol 26: 178–185. [CrossRef] [Google Scholar]
- Graham LE, Wilcox LW. 2000. Algae. Prentice-Hall, Inc., 640 pp. [Google Scholar]
- He NTT. 2012. Water environment quality and plankton diversity in Van Uc estuary. Master's thesis in Ecology. Hanoi University of Science, Vietnam. [Google Scholar]
- Herzig A. 1987. The analysis of planktonic Rotifres population. Hydrobiologia 147: 163–180. [Google Scholar]
- Hoanh CT, Guttuman H, Droogers P, Aerts J. 2003. Water, Climate, Food, and Environment in the Mekong basin in South Asia. Final report, contribution to the adaption strategies to changing environment ADAPT project. International Water Management Institute. [Google Scholar]
- Hung ND, Ngot PV, Toan Em QV, Thuy VTB. 2018. Survey of salinity of surface water and pore water in some mangrove vegetation along Tien river, Tien Giang province. J Natur Sci Technol Ho Chi Minh City Univ Educ 15: 156–169. [Google Scholar]
- Larsen J, Nguyen NL. 2004. Potentially Toxic Microalgae of Vietnamese Waters. Opera Botanica 140: 5–216. [Google Scholar]
- Lavens P, Sorgeloos P. 1996. Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper 361p. [Google Scholar]
- Li D, Long D, Zhao J, Lu H, Hong Y. 2017. Observed changes in flow regimes in the Mekong River basin. J Hydrol 551: 217–232. [CrossRef] [Google Scholar]
- Lien NTK, Giang HT, Ut VN. 2013. Diversity of zooplankton in Cu Lao Dung mangrove ecosystem in Soc Trang province. J Sci Can Tho Univ B 25: 149–157. [Google Scholar]
- Lien NTL, Gai DN, Giang HT, Ut VN. 2014. Zooplankton species composition in Hau River- from Hau Giang to Soc Trang, in the dry season. J Sci Can Tho Univ 2: 284–291. [Google Scholar]
- Long PH, Lauri H, Kummu M, Koponen J, van Vliet MTH, Supit I, Leemans R, Kabat P, Ludwig F. 2016. Mekong River flow and hydrological extremes under climate change. Hydrol Earth Syst Sci 20: 3027–3041. [Google Scholar]
- Ludwig GM. 1999. Zooplankton Succession and Larval Fish Culture in Freshwater Ponds. SRAC Publication No. 700. [Google Scholar]
- Matsuyama Y. 1999. Harmful effect of Dinoflagellate heterocapsa circularisquama on shellfish aquaculture in Japan. JARQ 33: 283–293. [Google Scholar]
- MRC. 2009. The flow of the Mekong. MRC Management Information booklet series No. 2. [Google Scholar]
- Nhan DK, Be NV, Trung NH. 2007. Water wse and competition in the Mekong delta, Vietnam. Challenges to sustainable development in the Mekong delta: regional and National Policy Issues and research Needs. Sustain Mekong Res Netw 143–188. [Google Scholar]
- Nielsen DL, Brock MA, Rees GN, Baldwin DS. 2003. The effect of increasing salinity on freshwater ecosystem in Australia. Aust J Bot 51: 655–665. [CrossRef] [Google Scholar]
- Paturej E, Gutkowska A. 2015. The effect of salinity levels on the structure of zooplankton communities. Arch Biol Sci Belgade 67: 483–492. [CrossRef] [Google Scholar]
- Porchas-Cornejo MA, Martínez-Córdova LR, Martínez-Porchas M, Barraza-Guardado R, Ramos-Trujillo L. 2013. Study of zooplankton communities in shrimp earthen ponds, with and without organic nutrient-enriched substrates. Aquacult Int 21: 65–73. [CrossRef] [Google Scholar]
- Prado P, Caiola N, Ibanez C. 2017. Water management alters phytoplankton and zooplankton communities in Ebro delta coastal lagoons. Limnetica 36: 113–126. [Google Scholar]
- Qian G, Zhaoli X, Ping Z. 2008. The relation between distribution of zooplankton and salinity in the Changjiang Estuary. Chin J Oceanol Limnol 26: 178–185. [Google Scholar]
- Quyen NTK, Amararatne K. 2016. The role of ecosystem services of the Hau River to the aquaculture and fishing community in Long Xuyen city An Giang province. J Sci Can Tho Univ 42: 75–84. [CrossRef] [Google Scholar]
- Rajashree S, Tapati D, Budhin G, Akash K, Vivekanand S, Debangshu DN. 2017. Community structure and monthly dynamics of zooplankton in high altitude rice fish system in Eastern Himalayan region of India. Int J Life Sci 5: 362–378. [Google Scholar]
- Raybaud V, Nival P, Mousseau L, Gubanova A, Altukhov D, Khvorov S, Ibanez F, Andersen V. 2008. Short term changes in zooplankton community during the summer-autumn transition in the open NW Mediterranean Sea: species composition, abundance and diversity. Biogeosciences 5: 1765–1782. [Google Scholar]
- Ruppert EE, Barnes RD. 1994. Invertebrate zoology. Brooks/Cole, Thomson Learning, 1056pp. [Google Scholar]
- Shirota A. 1966. The plankton of South Vietnam-Freshwater and marine plankton. Overseas Technical Cooperation Agency, Japan. [Google Scholar]
- Silva AMA, Barbosa JEL, Medeiros PR, Rocha RM, Lucea-Filho MA, Silva DF. 2009. Zooplankton (Cladocera and Rotifera) variations along a horizontal salinity gradient and during two seasons (dry and rainy) in a tropical inverse estuary (Northeast Brazil). Pan-Am J Aquat Sci 4: 226–238. [Google Scholar]
- Spoljar M, Drazina T, Kuczynska-Kippen N, Zhang C, Ternjej I, Kovacevic G, Lajtner J, Fressl J. 2018. Zooplankton traits in the water quality assessment and restoration of shallow lakes. 1st International Conference “The Holistic Approach to Environment” Sisak, September 13th–14th, 2018. [Google Scholar]
- Steinberg DK, Ruck KE, Gleiber MR, Garzio LM, Cope JS, Bernard KS, Stammerjohn SE, Schofield OM, Quetin LB, Ross RM. 2015. Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep Sea Res I 101: 54–70. [CrossRef] [Google Scholar]
- Sunada K. 2009. Study on Asian River Basin. CREST Asian River Basins: Water Policy Study Team. [Google Scholar]
- Tavsanoglu UN, Maleki R, Akbulut N. 2015. Effects of salinity on the zooplankton community structure in two Maar lakes and one freshwater lake in the Konya closed basin, Turkey. Ekoloji 24: 25–32. [Google Scholar]
- Toruan RL. 2012. Zooplankton community emerging from fresh and saline wetlands. Ecohydrol Hydrobiol 12: 53–63. [CrossRef] [Google Scholar]
- Tri LQ. 2016. The impact of climate change on agricultural production in the Mekong Delta. Vietnam J Sci Technol 8: 40–42. [Google Scholar]
- Tuan LA, Hoanh CT, Miller F, Sinh BT. 2007. Flood and salinity management in the Mekong delta, Vietnam. Challenges to sustainable development in the Mekong delta: regional and national policy issues and research needs: Literature analysis. Bangkok, Thailand: The Sustainable Mekong Research Network (Sumernet), 15–68. [Google Scholar]
- Valdes L, Moral M. 1998. Time series analysis of copepod diversity and species richness in the southern bay of Biscay off Santander, Spain in relation to environmental conditions. ICES J Mar Sci 55: 783–792. [Google Scholar]
- Van MV, Dinh TD, Tuan NA. 2012. Species composition and density of plankton distributed in the coastal areas from Soc Trang to Bac Lieu. J Sci Can Tho Univ 23a: 89–99. [Google Scholar]
- Vieira L, Azeiteiro U, Re P, Pastorinho R, Marques JC, Morgado F. 2003. Zooplankton distribution in a temperate estuary (Mondego estuary southern arm: Western Portugal). Acta Oecolog 24: 163–173. [CrossRef] [Google Scholar]
- Werba J. 2016. Zooplankton community structure and ecosystem function across a salinity gradient. Masteral Thesis. Faculty of Biology, East Carolina University. [Google Scholar]
- Zakaria HY, Radwan AA, Said AA. 2007. Influence of salinity variations on zooplankton community in El-Mex Bay Alexandria, Egypt. Egypt J Aquat Res 33: 52–67. [Google Scholar]
- Zorina-Sakharova K, Liashenko A, Marchenko I. 2014. Effects of salinity on the zooplankton communities in the Fore-Delta of Kyliya Branch of the Danube River. Acta Zool Bulg 7: 129–133. [Google Scholar]
Cite this article as: Nguyen CT, Vila-Gispert A, Quintana XD, Au VH, Nguyen TP, Vu NU. 2020. Effects of salinity on species composition of zooplankton on Hau River, Mekong Delta, Vietnam. Ann. Limnol. - Int. J. Lim. 56: 20
All Tables
List of 128 species belonging to four main zooplankton groups including Protozoa, Rotifera, Cladocera and Copepoda found in the estuarine areas of Hau River, Mekong Delta, Vietnam.
All Figures
 |
Fig. 1 Hau River and 3 sites (I, II, III) for zooplankton sampling. |
| In the text | |
 |
Fig. 2 Fluctuation of salinity on Hau River throughout sampling periods. |
| In the text | |
 |
Fig. 3 Species composition and density (figures in the bracket) of zooplankton on Hau River. |
| In the text | |
 |
Fig. 4 Cumulative Dominance (%) and Species rank of 4 zooplankton groups on Hau River. |
| In the text | |
 |
Fig. 5 Number of zooplankton species (5a), and % of appearance (5b) at different salinities |
| In the text | |
 |
Fig. 6 Fluctuation of zooplankton densities at different salinities. |
| In the text | |
 |
Fig. 7 PCA biplot of the Hellinger-transformed 21 zooplankton species under different salinities on Hau River (PC1 explained 24.8% var., PC2 explained 37.7% var.). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


