| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 56, 2020
|
|
|---|---|---|
| Article Number | 21 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/limn/2020019 | |
| Published online | 10 August 2020 | |
Research Article
Mouthpart morphology and food habits of a Pampean population of Cloeon dipterum (Linnaeus, 1761) (Ephemeroptera: Baetidae)
1
Universidad Nacional de Luján, Rutas 5 y 7, Luján, Buenos Aires, Argentina
2
Dpto. de Ciencias Básicas, Universidad Nacional de Luján. Instituto de Ecología y Desarrollo Sustentable (INEDES), CONICET-UNLu, Rutas 5 y 7, Luján, Buenos Aires, Argentina
* Corresponding author: rochaluciana17@yahoo.com.ar
Received:
22
August
2019
Accepted:
15
July
2020
Knowledge of the feeding habits of aquatic insects and assignation to different functional feeding groups contributes to a better comprehension of aquatic ecosystems. The feeding habits of larval stages (4–6 mm) of Cloeon dipterum (Linnaeus, 1761) were studied through mouthpart morphology, gut content and were tested in food particle size preference experiments. The description of the mouthparts consisted in the dissection of them and their observation in an optical microscope. Gut content analysis was carried out by ventral dissection of the thorax to isolate the digestive tract. The content of each larva was homogenized, mounted on slides and observed under an optical microscope at 400× magnification with a graduated eyepiece. Food preference experiments consisted on offering fine particulate organic matter (FPOM) and coarse (CPOM) leaves of Laurus nobilis simultaneously. Mouthparts are characterized by robust mandibles with well-developed and asymmetric molar surfaces and maxillae and labium with developed palps, with short setae. Gut content of C. dipterum was dominated by fine detritus represented by 76.9% (SD = 25.7) of the covered area. Also, in the food preference experiments was detected that FPOM consumption was greater than CPOM. Consequently, we consider that the larval stages of C. dipterum are functionally classified as collectors-gatherers preferring fine particle size, and secondary scrapers for CPOM manipulation.
Key words: Aquatic insects / aquatic ecosystems / Argentina / Cloeon dipterum / functional feeding group
© EDP Sciences, 2020
1 Introduction
Invertebrates carry out an essential role in aquatic ecosystems accelerating the decomposition of detritus and releasing nutrients in solution through their food activities, excretion and burrowing. Likewise, invertebrates are food sources for aquatic and overland consumers (Covich et al., 1999; Cummins et al., 2005) and they are structured along a gradient induced by different entry ways of organic matter, from headwater to downstream (Vannote et al., 1980; Bundschuh and McKie, 2015). Due to that the types of food resources and their availability are impacted for a continuous gradient of physical conditions, the information obtained of feeding habits study of aquatic insects allow the comprehension of processes that occur in lotic ecosystems (Cummins, 1973; Cummins et al., 2005).
Functional Feeding Groups (FFGs) were defined by Cummins (1973) for the Northern Hemisphere, based on the feeding mechanism, the type and size of food consumed. For Neotropical region, several authors agree that a considerable percentage of macroinvertebrates do not present the same feeding habits than respective species of Europe or North America (Tomanova et al., 2006). Therefore, a functional assignment of the present taxa in the neotropic is necessary (Cummins et al., 2005; Chará-Serna et al., 2010; Saigo et al., 2016).
Likewise, is known that several species may exhibit only one feeding habit during their development, but in other species the food consumed may change during ontogeny or seasonally (Stewart and Stark, 1988; Merritt and Cummins, 1996). Also, the longitudinal zonation of the availability of resources in the system allows invertebrates to move from one place to another in search of those resources, or to change the FFGs (Miserendino and Pizzolón, 2003).
To optimize the assignment of macroinvertebrates to the different FFGs and detect patterns of community structure, several authors have combined gut content analysis with the mouthpart morphology and behavior observations (Palmer and O'Keeffe, 1992; Palmer et al., 1993; Albariño, 2001; Guzmán-Soto and Tamarís-Turizo, 2014; Álvarez-Soraca et al., 2017).
In Argentina, knowledge of insect feeding habits in streams have been studied in some regions (Díaz Villanueva and Albariño, 1999; Albariño, 2001; Gil et al., 2006; Reynaga, 2009; Saigo et al., 2009; Reynaga and Rueda Martín, 2010), but it is still incomplete because little is known about local entomofauna.
Among aquatic insects, the order Ephemeroptera presents species of several FFGs according to the diverse feeding mechanisms that they have developed in the different regions that inhabit (Cummins et al., 1973; Domínguez et al., 2006; Flowers and De la Rosa, 2010), and they occupy several trophic levels because some are primary consumers and others are secondary consumers. Based on these considerations, we intend to contribute to the knowledge about the type and habit of feeding of Cloeon dipterum (Linnaeus, 1761), belonging to Baetidae, one of the most diverse and abundant families of the order Ephemeroptera in South America (Domínguez and Fernández, 2009). C. dipterum is one of the most common and abundant mayfly distributed throughout the temperate areas of Eurasia and in recent years it has been distributed in the American continent, particularly in North America (Canada and USA), and in South America (Chile and Argentina). The aim of this work was to establish the food habits of immature stages of C. dipterum (Baetidae: Ephemeroptera) from Buenos Aires (Argentina). Our working hypotheses were: (1) the immature stages (4–6 mm) of Cloeon dipterum consume more fine particulate organic matter (FPOM) than coarse particulate organic matter (CPOM) when are offered simultaneously; (2) The POM (FPOM and CPOM) is incorporated by collection and scraping mechanisms. These hypotheses were tested throughout the combination of the study of mouthparts morphology, laboratory experiments of food preference and gut content analysis.
2 Materials and methods
2.1 Collection of larvae and description of the head and mouthpart morphology
Larvae were collected in December (2017), January and March (2018) from two pools located in General Rodriguez (34°36′21′′ S 58°57′22″ W and 34°36′59″ S 58°55′17″ W) in the Northeast of Buenos Aires. Larvae were collected using a hand net of 1000 μm pore size. In situ, Populus sp., Pinus sp. and Laurus nobilis leaf litter were collected, which represents the main source of organic matter in the pools.
In the laboratory larvae were separated according to body size: 1–3 mm (small), 4–6 mm (intermediate) and greater than 6 mm (large).
To describe mouthpart we worked with 11 larvae of the last stage. The head was removed and the mouthparts were dissected. Each mouthpart was assembled in a permanent preparation with glycerin–gelatin (D́Ambrogio de Argüeso, 1986; Zarlavsky, 2014). Then, mouthparts were examined under a stereoscopic microscope (40×) and an optical microscope (100) and these were photographed in stereoscopic microscope Leica S8APO (80×) with a DFC295 camera and in optical microscope (100×) with a Canon PowerShot G10 digital camera with 5× optical zoom. In addition, mouthparts were measured to supplement the morphological description. For it, we used the ImageJ program (Rasband, 1997–2018) to estimate the morphometric measures (area, average width and average length) of each mouthpart ( Fig. 1).
2.2 Gut content analysis
Larvae were fixed in 4% formaldehyde and the observation of the gut content was carried out according to Palmer and O'Keeffe (1992) and Palmer et al. (1993). The head was removed and the ventral dissection of the thorax was performed to obtain the digestive tube of 90 larvae. The gut content of each larva was homogenized in bidistilled water, mounted on slides and observed under an optical microscope at 400× magnification. Eight food items were recognized: (1) fine particulate organic matter; (2) fungi; (3) mineral and inorganic matter; (4) animals remains; (5) algae (Chlorophyta); (6) diatoms; (7) vascular plant fragments; (8) others (three unidentified structures were included). The area covered by each item was measured with a graduated eyepiece and related to total area covered by gut content.
2.3 Laboratory experiments of food preference
2.3.1 Maintenance of the larvae in the laboratory
Each set of larvae (small, medium and large) was placed in a 4-liter white plastic container with non-chlorinated tap water and continuous oxygenation. Artificial plants of 7 cm of length were placed in each container to support the larvae, a maximum of 30 larvae and whole leaves of Populus sp., Pinus sp. and Laurus nobilis, as food source. The containers were covered with tulle to capture the emerged individuals and to facilitate their subsequent identification, the water was renewed every 15 days in order to remove the food remnants and prevent the excreted nitrogenous wastes from affecting the survival of the larvae.
The maintenance of the larvae and the experiments were carried out in the bioterium of the Ecology Lab under controlled conditions of ambient temperature, relative humidity and water temperature. Daily, the ambient temperature and relative humidity were controlled with a datalogger (LOG32) and the water temperature was recorded with a digital thermometer. During the study, the ambient temperature was 22 ± 1 °C, the relative humidity was 74 ± 2% and the water temperature was 19 ± 1°C.
2.3.2 Selection and acclimatization of the larvae
For the development of the experiments larvae at the intermediate stage were selected (we chose those larvae with a body length of 4–6 mm). The individuals were acclimatized for 24 hrs before each experiment in moderately hard reconstituted water (APHA, 2012) and were starved.
2.3.2.1 Experiment 1: Food preference between CPOM and FPOM.
The experiment was carried out in 4-liters plastic containers, containing 2.5 liters of moderately hard reconstituted water (APHA, 2012), with continuous aeration, at a temperature of 20 °C. Five units of 7-cm artificial plants were added and were used as a substrate for the larvae.
It was evaluated the preference of larvae of C. dipterum between two sizes of food particles offered simultaneously: CPOM and FPOM. The particulate material was obtained from the leaves collected in the pools. Leaves were washed and brushed to eliminate possible microorganisms adhered, and taken to stove at 60 °C until constant dry weight (DW). Then, they were ground and separated in CPOM (size greater than 1000 µm) and FPOM (size between 500 and 1000 µm) with sieves. Each portion of particulate material was immersed in moderately hard reconstituted water for 24 hrs before starting to the experiment, allowing the tissues to soften, fall to the bottom of the container and be available for larvae.
The experimental design consisted of one treatment and one control, with five replicates each. In each experimental unit 30 larvae and 25 mg of DW of each type of food (CPOM and FPOM) were placed, and in the control it was placed the same quantity of food as in the experimental unit. The experiment lasted 70 hrs and the larvae present were collected and counted; those that passed to the last stage (mature stage) in that period were excluded. Such exclusion is carried out because the larvae in the mature stage do not feed; the mouthparts are not functional (Domínguez & Fernández, 2009) and the associated muscles degenerate (Harker 1999). CPOM was collected with pin and FPOM was collected with Pasteur pipette. Then, FPOM was filtered with GF / C filters (previously weighed) and dried in an oven at 60 °C for 48 hrs to obtain the final DW. The consumption of each particle size was estimated by the DW difference.
2.3.2.2 Experiment 2: Production of FPOM as a result of larval activity
This experiment was to estimate FPOM production through mechanical breakage by larvae feeding CPOM. In each experimental unit 30 larvae and 25 mg of DW of CPOM were placed under the same conditions as in the experiment 1, for 70 hrs. The remaining CPOM and the FPOM produced were extracted separately and brought to stove at 60 °C for 48 hrs (final DW); they were weighed in the analytical scale and the CPOM consumption was calculated through of the initial and final DW difference. FPOM would correspond to the material produced by mechanical breakage of the larvae.
The estimation of the FPOM produced by the larvae by mechanical breakage of the CPOM avoided the overestimation of CPOM consumption and the underestimation of FPOM consumption.
2.4 Statistical analysis
Dietary variation was tested through a Kruskal–Wallis test for the covered item–area proportions obtained in gut contents. When significant differences were obtained, a Nemenyi test (p < 0.05) for multiple comparisons was applied (Zar, 1999). The statistical difference in larval consumption of FPOM and CPOM was tested through a nonparametric statistical analysis Kolmogorov-Smirnov. The statistical analyzes were carried out using software R version 3.4.1 (R Development Core Team, 2016) and STATISTICA 7.0 (StatSoft Inc., 2004).
3 Results
3.1 Description of the head and the mouthpart morphology of C. dipterum
The description of mouthparts corresponds to the analysis of 11 larvae of C. dipterum of an average length of 5.64 mm (±0.45 mm). The head of caramel coloration and sclerotized presented an average area of 4381.3 µm2 (±826.4 µm2); wider in the middle zone (hw2) than in the anterior (hw1) and posterior (hw3) ( Tab. 1). The antennae, located before compound eyes and ocelli, do not have setae and have three segments, which the third is multi-segmented. Females have compound eyes of black color that occupy the superior laterals of the head capsule (Fig. 1a). Males present two types of compound eyes: one type of black color, with similar position of the females and another one, dark brown, which occupies the greater part of the surface of the vertex until the coronal suture, called turbanate eye. The mouthparts are hypognathous and they are anteriorly enclosed by the clypeo-labral lobe.
The labrum of clear caramel color presents an average area of 373.1 µm2 (±17.3 µm2) and it is wider than long. In dorsal view, the anterior margin presents short bristles and a sharp indentation at the edge. In ventral view the inner surface presents setae.
The mandibles have three surfaces that articulate to the head capsule: articulatory surface anterior, lateral and posterior (Fig. 1a,c,d − blue arrows); they are very sclerotized and asymmetric. The internal margin has two cutting edges, one superior edge formed by the incisors (internal and external) and the other one formed by the molar surface. The prostheca is articulated in the base of the internal incisor, and between it and the molar surface there is a group of fine and short setae (mandibular setae). The prostheca of the left mandible is thicker than the right one. The molar surface of the left mandible has a row of thick teeth and blunt ends; the molar surface of the right mandible has a blade.
The hypopharynx forms the dorsal and posterior wall of the preoral cavity and it is constituted by the lingua and the superlinguas. The lingua of 211.7 µm2 (±27.7 µm2) area, tongue-like, is longer than wide, and has proximal and lateral bristles at the junction with the labrum (Fig. 1e). The superlinguae are flattened lobes of 206.3 µm2 area (± 19.9 µm2), which are located on both sides of the lingua and have thickened external lateral edges and fine bristles at the ends.
The labium has glossae and paraglossae with bristles in the edges and areas of 140.3 µm2 (±11.8 µm2) and 171.5 µm2 (±14.1 µm2), respectively (h). The labial palps present three segments of different length. In the second segment there are long and fine bristles in the upper half and in the third segment, the dorsal surface presents rows of thick bristles that increase in length towards the edges.
The maxillae are articulated laterally to the head capsule through the cardo and are formed by the cardo, the stipes, the galea–lacinia and the maxillary palps (Fig. 1f,g). The lacinia has two rows of bristles, anterior and posterior. The maxillary palps, approximately 2.5 times longer than the body (stipes and galea–lacinia), present three segments: the first two have similar areas and the third one approximately 1.48 times smaller than the first two (Tab. 1).
Variables measured in the different mouthparts of Cloeon dipterum (Linnaeus, 1761), with their respective acronyms. SD: standard deviation, MIN: minimum, MAX: maximum.
 |
Fig. 1 Mouthparts of Cloeon dipterum (Linnaeus, 1761) measured with their acronyms. a Head, dorsal view. b Labrum. c Right mandible, d Left mandible. e Hypopharynx. f Right maxilla. g Left maxilla. h Labium. hw: head width; hl: head long; lw: labrum width; ll: labrum long; rmdw: right mandible width; rmdl: right mandible long; lmdw: left mandible width; lmdl: left mandible long; hsw: hypopharynx superlingua width; hsl: hypopharynx superlingua long; liw: lingua width; lil: lingua long; mpw: maxillary palp width; mpl: maxillary palp long; gw: glossae width; gl: glossae long; pw: paraglossae width; pl: paraglossae long; lpw: labial palp width; lpl: labial palp long. The blue arrows indicate the articulatory surfaces. |
3.2 Gut content analysis
Of the 90 larvae dissected 8 empty guts were found. Of the eight dietary items established for the diet (FPOM, fungi, mineral and inorganic matter, animals remains, algae, diatoms, vascular plant fragments; Fig. 2), FPOM were present in 100% of the samples and it occupied 76.9% (SD = 25.7) of the coverage area. This item was significantly more abundant than the rest (Kruskal-Wallis, Nemenyi p < 0.05) ( Fig. 3). Chlorophyta algae were present in 39% of the samples and it occupied an area of 7.6% (SD = 16.2). Diatoms occupied 4% (SD = 12.0) of the covered area and were present in 34% of the gut contents. Fungi and mineral and inorganic matter occupied similar coverage area (3.2% (SD = 5.8) and 3.1% (SD = 6.3), respectively); fungi were present in 88% of the samples, included hyphae and asexual reproduction structures (conidia), Alternaria sp. (Ascomycota) was a very common fungus in the guts, Vascular plants fragments (VPF) and animals remains (AR) had the lowest coverage. VPF occupied 2.5% (SD = 5.0) of the area and was present in 57% of the gut content; epidermal cells, conduction tissue and pollen grains were observed. In AR, fragments of exoskeleton, segments, setae and nematodes were recognized. In the item other, three unidentified structures were included that occupied 0.5% (SD = 0.9).
 |
Fig. 2 Food items found in gut contents of Cloeon dipterum. a. FPOM (fine particulate organic matter); b. Fungi; c. Mineral material; d. Animal remains; e. Chlorophyta algae; f. Diatom; g. Vascular plant epidermal cells on the left and conduction tissue (xylem) on the right. |
 |
Fig. 3 Dietary composition of Cloeon dipterum (Linnaeus 1761) larvae. FPOM: fine particulate organic matter; F: fungi; MIM: mineral and inorganic material; AR: animals remains; A: Chlorophyta algae; D: diatoms; VPF: vascular plants fragments; O: others. Error bar: SD. |
3.3 Laboratory experiments of food preference
In the experiments of food preference where FPOM and CPOM were offered simultaneously, we detected that the FPOM consumption was higher than CPOM (0.013 (±0.004) mg (mean ± SD) DW of FPOM larva‑1 day‑1 and 0.010 (±0.006) mg DW of CPOM larva‑1 day‑1, respectively) (Kolmogorov–Smirnov p > 0.05) ( Fig. 4). Also, it was observed that the organisms manipulated the CPOM and produced FPOM.
Experiment 2 showed that 62.2% DW loss of CPOM corresponded to ingestion by organisms. The remaining percentage (37.8%) corresponded to a secondary conversion of CPOM to FPOM through mechanical breakage.
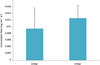 |
Fig. 4 Larval consumption rate (as mg of food consumed per individual per day) of Cloeon dipterum (Linnaeus 1761) larvae in the feeding experiment. Error bar = SD. Kolmogorov–Smirnov test (p > 0.05). |
4 Discussion
The present study aimed to establish the food habits of the immature stage of Cloeon dipterum (Baetidae: Ephemeroptera) from Buenos Aires (Argentina). The results support the hypotheses that the immature stages of Cloeon dipterum prefer FPOM and obtain it by collecting and scraping mechanisms. We assume that these feeding mechanisms are carried out by the maxillae, the labium and the mandibles. Gut contents of the larvae examined showed a clear predominance of FPOM, observation that was also recorded in laboratory food preference experiments.
Brown (1961b) also found a higher percentage of fine detritus in the gut contents of larvae between 4 and 6 mm size as those analyzed here. Likewise, Brown (1960) and Palmer et al. (1993) observed that larger individuals manipulated and ingested larger food fragments, and that smaller organisms consumed smaller fragments.
Although eight food items were found in the diet of the 82 larvae, the sum of fungi, mineral and inorganic matter, animals remains, algae, diatoms, vascular plant fragments and others did not exceed 24% of the coverage area (Fig. 3). Alternaria sp. (Fungi) was present in 88% of the observed individuals but in a low proportion (3.2% of the coverage area). We assume that the consumed leaves could be colonized by this fungus, given that Alternaria is a widely distributed genus in the soil and decomposing organic matter, and affects the plants as a result of phytotoxin synthesis (Pavón Moreno et al., 2012). Also, the larvae consumed unicellular algae and diatoms (recorded in 39% and 41% of the individuals, respectively), organisms that are part of the periphyton, so we presume that the fungi and algae present in the gut content were obtained by scraping the leaves through the movement of the molar surfaces of the mandibles and robust wedge-shaped incisors, as Sroka (2009) states that the mandibles not only have a role in the grinding of food. Fragments of invertebrates and mineral and inorganic material could accidentally be ingested when the larvae feed on the particulate material, as Cummins (1973) refers to foliar material, which would explain its presence in a high percentage of the individuals observed (73% and 95%, respectively), but in low proportion in the gut contents (Fig. 3). Among the consumed invertebrates, very small fragments of exoskeletons and appendages were recognized, as well as complete nematodes that did not exceed 200 µm. The differences observed in the proportions of the intake could be linked to three factors: (1) the availability of food items in the habitat (Allan et al., 1987; Tomanova, 2006); (2) the intensity of consumption, which fluctuates with seasonality, and therefore with temperature (Brown, 1961b); (3) the size of the larvae (Brown, 1960; Albariño, 2001).
In laboratory experiments of food preference, Cianciara (1980) and Ivanova (1958) observed that larvae of C. dipterum between 4 and 6.5 mm in length preferred to consume algae, and those of larger sizes (>6.5 mm to 8.8 mm) preferred coarse organic detritus. In our experiment we detected that C. dipterum (medium size 4–6 mm) preferred the fine particulate material (Fig. 4). Although it was also fed with coarse material, our observations showed the handling of CPOM and production of FPOM by the larvae in the laboratory, this behavior could be due to C. dipterum has mouthparts adapted to consume both FPOM and CPOM (Brown, 1961a). According to these observations and, taking into account the absence of CPOM in the gut content and a possible tendency to obtain food by scraping, supported by the presence of fungi, unicellular algae and diatoms in the gut content, we could assume that there was a transformation of coarse to fine organic material by CPOM scraping and subsequent grinding.
The mouthparts presented characteristics adapted to the collection and scraping, and are very similar to those of Cloeon simile (Eaton 1870), recorded mainly in Europe (Sowa, 1980; Puig et al., 1986; Cayrou and Cereghino 2003), with habits scrapers collectors. On the other hand, C. dipterum presented differences mainly in the labial and maxillary palps, with respect to species of Callibaetis (Family Baetidae) such as C. sellaki (Weyenbergh 1883), C. guttatus (Navás 1915) and C. willineri (Navás 1932), recorded in Buenos Aires (Argentina). Cloeon dipterum labial and maxillary palps are constituted by three segments and the end of the third labial segment has a quadrangular shape unlike the Callibaetis species in which their labial and maxillary palps have two segments and the end of the second labial segment is spoon-shaped. The species of Callibaetis, according to Cummins et al. (2005), are collectors-gatherers. We assume that in C. dipterum, the maxillae and the labium, provided with short setae and elongated palps, are the parts involved in the collection of the fine material available, which contributed to the manipulation, collection and ingestion of the food. This coincides with Brown (1961a) who observed that the labial palps and lacinia are used to manipulate the fine detritus, and that the mandibles did not participate in the collection. Also, he observed that, when C. dipterum consumed filamentous algae or vascular vegetal tissue, the mandibles had a more important role in the collection of food. C. dipterum presents robust wedge-shaped incisors and molar surfaces of the left mandible with a row of thick teeth and robust which, according to Baptista et al., 2006, they are structures specialized for scraping the periphyton.
According to our observations of the mouthparts and the gut content, as well as the laboratory preference experiments, the larvae C. dipterum (4–6 mm) behaved mainly as a collector-gatherer, which preferentially consume FPOM, and secondarily as a scraper.
Acknowledgment
The authors are grateful to R. Albariño for his kind support of the experimental work. We thank F. Momo, C. Coviella and D. Yanega for their suggestions. We thank Universidad Nacional de Luján personnel for their access and field assistance to some of the study sites. Also, we thank T. Poretti, J. Mendoza, M.E. García and A. Fañani for their assistance in the field and lab work. This work was supported by the project PI4 (DISPCD-CBLUJ:0000503-18), Universidad Nacional de Luján.
References
- Albariño RJ. 2001. The food habits and mouthpart morphology of a South Andes population of Klapopteryx kuscheli (Plecoptera: Austroperlidae). Aquat Insects: Int J Freshw Entomol 23: 171–181. [CrossRef] [Google Scholar]
- Allan JD, Flecker AS, McClintock NL. 1987. Prey preference of stoneflies: sedentary vs mobile prey. Oikos 49: 323–331. [Google Scholar]
- Álvarez-Soraca KD, Tamaris-Turizo CE, Guzmán-Soto CJ. 2017. Morfología de las piezas bucales y hábitos alimenticios de Leptonema y Smicridea (Trichoptera: Hydropsychidae) del río Gaira, Sierra Nevada de Santa Marta, Colombia. Rev Biol Trop 65: 1231–1245. [Google Scholar]
- APHA. 2012. American Public Health Association, American Water Works Association, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 541 p. [Google Scholar]
- Baptista DF, Buss DF, Dias LG, Nessimian JL, Da Silva ER, Neto ADM, Andrade LR. 2006. Functional feeding groups of Brazilian Ephemeroptera nymphs: ultrastructure of mouthparts. Ann Limnolog Int J Limnol 42: 87–96. [CrossRef] [Google Scholar]
- Brown DS. 1960. The ingestion and digestion of algae by Chloeon dipterum L. (Ephemeroptera). Hydrobiologia 16: 81–96. [Google Scholar]
- Brown DS. 1961a. The morphology and function of the mouthparts of Cloeon dipterum L. and Baetis rhodani (Pictet) (Insecta, Ephemeroptera). Proc Zool Soc Lond 136: 147–176. [CrossRef] [Google Scholar]
- Brown DS. 1961b. The food of the larvae of Cloeon dipterum L. and Baetis rhodani (Pictet) (Insecta, Ephemeroptera). J Anim Ecol 30: 55–75. [Google Scholar]
- Bundschuh M, McKie BG. 2015. An ecological and ecotoxicological perspective on fine particulate organic matter in streams. Freshw Biol 61: 2063–2074. [Google Scholar]
- Cayrou J, Cereghino R. 2003. Life history, growth and secondary production of Caenis luctuosa and Cloeon simile (Ephemeroptera) in a small pond, SW France. Aquat Insects 25: 191–201. [Google Scholar]
- Chará-Serna AM, Chara JD, Zúñiga M, Pedraza GX, Giraldo LP. 2010. Clasificación trófica de insectos acuáticos en ocho quebradas protegidas de la ecorregión cafetera colombiana. U Scient 15: 27–36. [Google Scholar]
- Cianciara S. 1980. Food preference of Cloeon dipterum (L) larvae and dependence of their development and growth on the type of food. Pol Arch Hydrobiol 27: 143–160. [Google Scholar]
- Covich AP, Palmer MA, Crowl TA. 1999. The role of benthic invertebrate species in freshwater ecosystems. BioScience 49: 119–127. [Google Scholar]
- Cummins KW. 1973. Trophic relations of aquatic insects. Annu Rev Entomol 18: 183–206. [Google Scholar]
- Cummins KW, Merrit RW, Andrade PC. 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud Neotrop Fauna Environ 40: 69–98. [CrossRef] [Google Scholar]
- D́Ambrogio de Argüeso A. 1986. Manual de técnicas en histología vegetal. Buenos Aires: Hemisferio Sur, 83 p. [Google Scholar]
- Díaz Villanueva V, Albariño RL. 1999. Feeding habit of Notoperla archiplatae (Plecoptera) larvae in a North Patagonia Andean stream, Argentina. Hydrobiologia 412: 43–52. [Google Scholar]
- Domínguez E, Molineri C, Pescador ML, Hubbard MD, Nieto C. 2006. Ephemeroptera of South America. In: Adis J, Arias JR, Rueda-Delgado G, Wantzen KM (Eds.), Aquatic Biodiversity in Latin America (ABLA). Vol. 2. Sofia-Moscow, Pensoft, 646 p. [Google Scholar]
- Domínguez E, Fernández HR. 2009. Macroinvertebrados bentónicos sudamericanos: sistemática y biología. 1er Ed. Tucumán, Fundación Miguel Lillo, 654 p. [Google Scholar]
- Flowers RW, De la Rosa C. 2010. “Ephemeroptera”. Rev Biol Trop (Int J Trop Biol) 58: 63–93. [Google Scholar]
- Gil MA, Garelis PA, Vallania EA. 2006. Hábitos alimenticios de larvas de Polycentropus joergenseni Ulmer, 1909 (Trichoptera: Polycentropodidae) en el Río Grande (San Luis, Argentina). Gayana 70: 206–209. [Google Scholar]
- Guzmán-Soto CJ, Tamarís-Turizo CE. 2014. Hábitos alimentarios de individuos inmaduros de Ephemeroptera, Plecoptera y Trichoptera en la parte media de un río tropical de montaña. Rev Biol Trop 62: 169–178. [PubMed] [Google Scholar]
- Harker JE. 1999. The structure of the foregut and midgut of nymphs, subimagos and imagos of Cloeon dipterum (Ephemeroptera) and the functions of the gut of adult mayflies. J Zool 248: 243–253. [Google Scholar]
- Ivanova SS. 1958. Nutrition of some mayfly larvae. Proc. Mikoyan Moscow tech. Inst. Fish Ind 9: 102–120. [Google Scholar]
- Miserendino ML, Pizzolón LA. 2003. Distribution of macroinvertebrate assemblages in the Azul-Quemquemtreu river basin, Patagonia, Argentina. N Z J Mar Freshw Res 37: 525–539. [CrossRef] [Google Scholar]
- Palmer CG, O'Keeffe JH. 1992. Feeding patterns of four macroinvertebrate taxa in the headwaters of the Buffalo River, Eastern Cape. Hydrobiologia 228: 157–173. [Google Scholar]
- Palmer CG, O'Keeffe JH, Palmer A, Dunne T, Radloff S. 1993. Macroinvertebrate functional feeding groups in the middle and lower reaches of the Buffalo River, Eastern Cape, South Africa. I. Dietary variability. Freshw Biol 29: 441–453. [Google Scholar]
- Pavón Moreno MA, González Alonso I, Martín de Santos R, García Lacarra T. 2012. Importancia del género Alternaria como productor de micotoxinas y agente causal de enfermedades humanas. Nutr Hospital 27: 1772–1781. [Google Scholar]
- Puig MA, Ferreras-Romero M, Rojas AMG. 1986. Ecosistemas de ríos temporales: ecología de las poblaciones de efemerópteros de la cuenca del río Bembézar (Sierra morena). Anal Biol 8: 65–69. [Google Scholar]
- Rasband WS. 1997–2018. National Institutes of Health, Bethasda, Maryland, USA. Image J, U.S. http://imagej.nih.gov/ij. [Google Scholar]
- R Development Core Team. 2016. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.R-project.org. [Google Scholar]
- Reynaga MC. 2009. Hábitos alimentarios de larvas de Trichoptera (Insecta) de una cuenca subtropical (Tucumán, Argentina). Ecol Austr 19: 207–214. [Google Scholar]
- Reynaga MC, Rueda Martín P. 2010. Trophic analysis of two species of Atopsyche (Trichoptera: Hydrobiosidae). Limnologica 40: 61–66. [Google Scholar]
- Saigo M, Marchese M, Montalto L. 2009. Feeding habits of Hyalella curvispina Shoemaker, 1942 (Amphipoda: Gammaridea) in floodplain lakes of Middle Paraná River. Nat Neotrop 40: 43–59. [Google Scholar]
- Saigo M, Marchese M, Wantzen K. 2016. A closer look at the main actors of Neotropical floodplain food webs: functional classification and niche overlap of dominant benthic invertebrates in a floodplain lake of Paraná River. Iheringia. Série Zool 106: 1–8. [Google Scholar]
- Sroka P. 2009. Morphology and ultrastructure of the molar area in the mandible of mayfly (Ephemeroptera) larvae. Aquat Insects: Int J Freshw Entomol 31: 471–484. [CrossRef] [Google Scholar]
- Sowa R. 1980. Taxonomy and ecology of European species of the Cloeon simile Eaton group (Ephemeroptera: Baetidae). Insect Syst Evol 11: 249–258. [Google Scholar]
- StatSoft Inc. 2004. STATISTICA (data analysis software system), version 7 http://www.statsoft.com. [Google Scholar]
- Stewart KW, Stark BP. 1988. Nymphs of North American stonefly genera (Plecoptera). The Thomas Say Foundation Entomological Society of America Series, 460 p. [Google Scholar]
- Tomanova S, Goitia E, Helešic J. 2006. Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia 556: 251–264. [Google Scholar]
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. 1980. The River Continuum Concept. Can J Fish Aquat Sci 37: 130–137. [Google Scholar]
- Zar JH. 1999. Biostatistical analysis. New Jersey: Prentice Hall, 663p. [Google Scholar]
- Zarlavsky GE. 2014. Histología Vegetal: técnicas simples y complejas. Soc Argent Botán, 198 p. [Google Scholar]
Cite this article as: Banegas BP, Casset MA, Silvera A, Rocha L. 2020. Mouthpart morphology and food habits of a Pampean population of Cloeon dipterum (Linnaeus, 1761) (Ephemeroptera: Baetidae). Ann. Limnol. - Int. J. Lim. 56: 21
All Tables
Variables measured in the different mouthparts of Cloeon dipterum (Linnaeus, 1761), with their respective acronyms. SD: standard deviation, MIN: minimum, MAX: maximum.
All Figures
 |
Fig. 1 Mouthparts of Cloeon dipterum (Linnaeus, 1761) measured with their acronyms. a Head, dorsal view. b Labrum. c Right mandible, d Left mandible. e Hypopharynx. f Right maxilla. g Left maxilla. h Labium. hw: head width; hl: head long; lw: labrum width; ll: labrum long; rmdw: right mandible width; rmdl: right mandible long; lmdw: left mandible width; lmdl: left mandible long; hsw: hypopharynx superlingua width; hsl: hypopharynx superlingua long; liw: lingua width; lil: lingua long; mpw: maxillary palp width; mpl: maxillary palp long; gw: glossae width; gl: glossae long; pw: paraglossae width; pl: paraglossae long; lpw: labial palp width; lpl: labial palp long. The blue arrows indicate the articulatory surfaces. |
| In the text | |
 |
Fig. 2 Food items found in gut contents of Cloeon dipterum. a. FPOM (fine particulate organic matter); b. Fungi; c. Mineral material; d. Animal remains; e. Chlorophyta algae; f. Diatom; g. Vascular plant epidermal cells on the left and conduction tissue (xylem) on the right. |
| In the text | |
 |
Fig. 3 Dietary composition of Cloeon dipterum (Linnaeus 1761) larvae. FPOM: fine particulate organic matter; F: fungi; MIM: mineral and inorganic material; AR: animals remains; A: Chlorophyta algae; D: diatoms; VPF: vascular plants fragments; O: others. Error bar: SD. |
| In the text | |
 |
Fig. 4 Larval consumption rate (as mg of food consumed per individual per day) of Cloeon dipterum (Linnaeus 1761) larvae in the feeding experiment. Error bar = SD. Kolmogorov–Smirnov test (p > 0.05). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


