| Issue |
Int. J. Lim.
Volume 58, 2022
|
|
|---|---|---|
| Article Number | 8 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/limn/2022009 | |
| Published online | 04 August 2022 | |
Research Article
Class III peroxidase and polyphenol oxidase activities in aquatic macrophytes during vegetative period in Bardača a wetland
1
Department of Biology, Faculty of Natural Sciences and Mathematics, University of Banja Luka, Mladena Stojanovića 2, 78000 Banja Luka, the Republic of Srpska, Bosnia and Herzegovina
2
Department of Chemistry, Faculty of Natural Sciences and Mathematics, University of Banja Luka, Mladena Stojanovića 2, 78000 Banja Luka, the Republic of Srpska, Bosnia and Herzegovina
* Corresponding author: tanja.maksimovic@pmf.unibl.org
Received:
7
March
2022
Accepted:
23
May
2022
In this study, changes in Class III peroxidase (POX) and polyphenol oxidase (PPO) activity in Phragmites australis (Cav.) Trin. Ex Steud. Utricularia vulgaris L. and Salvinia natans (L.) from the Bardača wetland during one vegetation season (June-October) were monitored. The highest activities of soluble and ionic cell wall bound peroxidases (solPOX and ionPOX, respectively) were measured for Phragmites communis (leaf > root > rhizome), followed by Utricularia vulgaris (whole plant), then Salvinia natans (whole plant). The results showed that during the vegetation period (August-September) the activity of solPOX, ionPOX and PPO in Phragmites communis increased, but the activity decreased drastically in October. For Salvinia natans and Utricularia vulgaris, a different seasonal distribution was obtained in the PPO activity, i.e. with a maximum activity during July and a minimum one during September. Different seasonal trends in enzyme activities are probably the result of abiotic stress caused by changing physic-chemical environmental conditions and different adaptive capacities of the studied species to habitat conditions. Correlations between physicochemical environmental parameters and enzyme activities indicate the possibility of using POX and PPO activities as an important bioindicatos of environmental status.
Key words: Peroxidases / polyphenol oxidase / aquatic macrophytes / oxidative stress
© EDP Sciences, 2022
1 Introduction
All year long, plants in natural habitats are exposed to different abiotic stress such as: increased UV-B radiation, floods, high salinity, extreme temperatures, hypoxia, nutrient deficiencies, toxic metals, herbicides, fungicides and others (Ali and Alqurainy, 2006; Ferreres et al., 2011; Hossain et al., 2012). Given that plants are sessile and unable to avoid stress actively and are constantly exposed to negative environmental conditions, the influence of one or more stressors leads to disruption of normal metabolic processes, disorders of homeostasis throughout the organism (Ali and Alqurainy, 2006). One of the consequences of plant exposure to different types of stress is oxidative stress resulting from increased production of reactive oxygen species [ROS: superoxide anion radical (O2 ._), hydrogen peroxide (H2O2), hydroxyl radical (OH.)]. Oxidative stress can lead to oxidative damage to cellular macromolecules and the cell itself. Plants possess a complex antioxidative system, consisting of non-enzymatic and enzymatic components, which protects the plant from oxidative damage caused by environmental stresses (Ali and Alqurainy, 2006; Chen et al., 2007). Although they do not represent the first line of defence against reactive oxygen species, Class III peroxidase (POX) and polyphenol oxidase (PPO) are pertinent antioxidant enzymes. Plant peroxidases are localized intracellularly (in vacuoles and cytoplasm) and extracellularly (apoplast and cell wall) (Takahama, 2004; Ferreres et al., 2011; Kukavica et al., 2012). Intracellular and apoplastic peroxidases are soluble, while ionic or covalent peroxidases are bound to the cell wall (Kukavica et al., 2012). They are involved in various metabolic processes with an important role in the process of growth and differentiation, auxin catabolism, cell wall protein crosslinking, phenolic compound oxidation, and are responsible for cell wall lignification, suberin synthesis, and senescence (aging) (Almagro et al., 2009; Dorantes et al., 2012; Kukavica et al., 2012). Therefore, changes in POX activity are used as sensitive biomarkers of abiotic and biotic stresses and their influence on plants: exposure to pathogens and chemicals (heavy metals, herbicides), increased light intensity, mechanical injuries (Takahama and Oniki, 2000; Ali and Alqurainy, 2006; Maksimović et al., 2020a). Most Class III peroxidases are excreted in the apoplast and are involved in two processes: reduction of H2O2 metabolites in the lignin synthesis (Hiraga et al., 2001) and the formation of reactive oxygen species.
PPO plays a key role in the regulation of auxin levels in plant tissues (Dorantes et al., 2012 and Zuniga, 2012), in the biosynthesis of plant pigments and lignins, and in the defence reactions of plants to pathogens. Polyphenol oxidase activity also depends on the chemical environment such as oxygen, phenolic compound content and pH levels (Mishra and Gautam, 2016). PPO activity is commonly increased in wounded tissues, and is therefore often considered a plant defence enzymes (Constabel and Barbehenn, 2008; Mishra and Gautam, 2016).
There is little information on the influence of environmental factors on POX and PPO of aquatic macrophytes. The changes in activities of POX and PPO may indicate intensity of natural long-term environmental stressors. The aim of this study was to examine the changes in the activities of POX and PPO of selected water macrophytes: Phragmites communis (Trin.) as an emergent plant, Utricularia vulgaris (L.) as submerged (unrooted) and Salvinia natans (L.) All. as floating (unrooted) plants.
2 Material and methods
2.1 Research area
The Bardača area is a unique wetland-marsh ecosystem created by the interaction of several natural and anthropogenic factors. In 1969, as a special nature reservation, Bardača was established as an area of great natural values and sensitive ecosystems, within which the production of cyprinid fish is predominant. Since February 2007, Bardača has been registered as a wetland of world importance by the Ramsar Convention and has been on the list of Important Bird Areas (IBA). Bardača covers the territory of 2810 ha (810 ha of aquatic and 2000 ha of terrestrial ecosystems). The research was conducted at the following sites: Necik Basin − located in the centre of the pond complex, with an area of 40 ha, average depth of 110 cm. The sampling point was located at 45° 06′ 47.7″ north latitude and 17° 27′ 04.7″ east longitude. The Sinjak basin covers the northernmost edge of the wetland, with an area of 40 ha and an average depth of 180 cm. The sampling point was located at 45° 06′ 55.1″ north latitude and 17° 26′ 02.9″ east longitude (Đurić et al., 2004).
2.2 Sampling
Sampling was performed once a month from June to October for P. communis, while S. natans and U. vulgaris were collected from June to September due to the end of their vegetative season. Sampling was done in the littoral zone at three different sampling points for both sites (three biological replicas). For each sampling point and for each examined plant species, a mixed sample containing 5–10 plants was prepared. The samples of P. communis were divided into root, rhizome and leaf, while S. natans and U. vulgaris were analyzed as whole plants.
2.3 Determination of physic-chemical characteristics of water
The physico-chemical analysis of water and collection of plant material were performed simultaneously. The temperature of water and air was determined by a mercury thermometer in the morning. Water transparency was determined using a Secchi disc. Oxygen concentration was determined using a Hach HQ30 flexi oximeter, the Eutech Cyberscan PC 10 pH meter was used for pH value and electrical conductivity, and the Eutech TN-100 turbidimeter was used to determine turbidity. Samples were always collected at the same profile points once a month and measurements were performed in a layer of water 30–50 cm below the surface. The samples were then transported on ice at a temperature of up to +4 °C and their analysis was performed within 24 h.
2.4 Extraction of proteins from plant tissue
After sampling, fresh plant material was drained, washed with distilled water, dried with paper towels, packed in aluminium foil bags, labelled, and then placed in a container with liquid nitrogen. To obtain the protein extract, the plant tissue was powder with liquid nitrogen and then homogenized with extraction buffer (100 mM Na-phosphate buffer (NaPi) pH 6.4 containing 1 mM PMSF (phenylmethanesulfonylfluoride) and 0.1% TWEEN. The plant tissue/extraction buffer ratio (w:v) varied used was different depending on the plant species (1 g : 4 mL for Phragmites communis; 1 g : 3 mL for Utricularia vulgaris; 1 g : 2 mL for Salvinia natans). The supernatant obtained after centrifugation at 3000 rpm for 15 min was used to determine the activity of soluble POX. For the purpose of extraction of ionic POX, the residue remaining after separation of the soluble protein was washed twice with 100 mM Na Pi pH 6.4. After washing, the residue was homogenized in 100 mM Na Pi containing 1 M NaCl. After 30-minute incubation at 4 °C, the homogenate was centrifuged at 3000 rpm for 15 min.
Plant tissue (0.2 g) and 2 mL of the same extraction buffer as for solPOX were used for PPO extraction. The homogenates were centrifuged at 3000 rpm for 15 min, and the resulting supernatant was used to determine the protein concentration and activity of the soluble PPO. Protein content was determined by the Lowry et al. (1951) method.
2.5 Determination of specific POX activity
The activity of solPOX and ionPOX was determined spectrophotometrically (Shimadzu UV-160, Kyoto, Japan) using pyrogallol as a substrate according to the modified method of Teisseire and Guy (2000). The reaction mixture contained 100 mM Na-phosphate buffer pH-6.4, 17 mM pyrogallol and different sample volumes depending on the plant species. The reaction was initiated by the addition of 4 mM hydrogen peroxide. The change in absorbance at 430 nm was monitored for one minute. The specific activity of solPOX and ionPOX was expressed as the amount of purpurogallin formed in units µmol −1 mgprot −1 min−1 using ε = 12 mM−1 cm−1 for purpurogallin.
2.6 Determination of specific PPO activity
The specific activity of PPO was determined spectrophotometrically by monitoring the increase in absorbance at 420 nm (A 420, ε = 12 mM−1 cm−1) for 10 min. The reaction mixture (3 mL) contained 100 mM Na-Pi buffer, pH 6, 67 mM pyrogallol and 100 μL of sample. Enzyme activity is expressed in µmol −1 mgprot −1 min−1.
2.7 Statistics
All data were statistically processed in the SPSS (Statistical Package for the Social Sciences) program. The analyses were performed in three independent replicates, and the analysed parameters were processed by the method of analysis of variance (ANOVA) of factorial examination, descriptive statistics and comparison of the obtained data. The LSD test for significance level p < 0.05 was used to compare the results and examine the intergroup differences from the ANOVA analysis within the Post hoc tests. All results are presented as mean values of three replicates ± standard error (SE).
3 Results
During the research period, the water temperature values at the Necik site varied in accordance with the air temperature in the range from 9.6 °C in October to 31.2 °C as measured in July ( Tab. 1). In contrast to the Necik, the Sinjak had slightly higher water temperature, with the highest value during July (31.1 °C) and the lowest during October (15.5 °C). During the research period, two peaks for oxygen concentration were detected (Tab. 1). The first peak was in the spring, with highest oxygen content, while the second one was in the summer with the lowest O2 content measured. According to the Decree on Water Classification and Categorization of Watercourses of Republika Srpska (Službeni glasnik Republike Srpske, 2001), at both Necik and Sinjak sites the water in the spring belonged to classes I and II with high saturation values indicating high organic production. On the other hand, in the summer there was a deficit of oxygen which suggested the class IV water quality, and in the autumn water belonged to class III. In the investigated sites during the vegetation period, due to intensive photosynthetic processes, carbonic acid balance was disturbed, which resulted in an increase in pH value (8–8.35) at the beginning of the season and a decrease during the summer period (Tab. 1). Electrical conductivity values ranged from 400 µS cm−1 in July to a maximum of 504 µS cm−1 in June, indicating a moderate load of dissolved water ions. Water transparency in the studied sites varied in the range of 28–70 cm, and according to the classification of lake trophic (OECD − Organization for Economic Cooperation and Development, 1982), the studied sites belong to hypertrophic water basins. The highest turbidity values, 27.60 NTU and the lowest water transparency of 28 cm were measured in August and September (Tab. 1).
Physico-chemical parameters of water at the investigated sites (Necik and Sinjak).
3.1 Activity of solPOX, ionPOX and PPO in aquatic macrophytes
The obtained results showed significant seasonal variations in the activity of solPOX and ionPOX as well as changes in relation to the site in the studied macrophytes. The activity of solPOX in the root of P. communis had a declining trend from June until October at both sites ( Fig. 1A). On the other side, the solPOX activity in the leaves of P. communis at site Sinjak significantly increased during the coming months, except the October, when the lowest activity at both sites was observed (Fig. 1B). The activity of solPOX in the rhizome of P. communis was significantly higher during July and August at Necik site (Fig. 1C).
In the P. communis leaves, ionPOX activity was significantly lower at both sites compared to root and stem activities. On the other hand, the lowest root ionPOX activity was measured at Sinjak site in October while ionPOX rhizome activity was highest in October at both sites ( Fig. 2A).
The activity of solPOX in S. natans at Sinjak site had a declining trend during the entire research period, so the lowest activity was measured at both sites in September ( Fig. 3A). The activity of ionPOX of S. natans was significantly higher during July and August at both sites (Fig. 3B).
In Utricularia vulgaris, an increase in activity in terms of aging (August-September) of both soluble and ionic peroxidases was observed, while minimal activity was measured at the beginning of the vegetation period ( Fig. 4).
The polyphenol oxidase activity during the vegetation period varied depending on the species, site, and sampling period. In the root of P. communis, a declining trend of PPO activity was observed at Necik site from June until September, while its activity significantly increased in October ( Fig. 5A). In July, at Sinjak site significant reduction of PPO activity in the root of P. communis was detected, while the highest value in the same sample was observed in October (Fig. 5A). Significant reduction of PPO activities in the leaves of P. communis was measured at Necik site in August, as well as at Sinjak site in July, while in autumn (September–October) its activity significantly increased (Fig. 5B). In the rhizome of P. communis the activity of PPO at both sites had a declining trend from July until September, while its increase in October (Fig. 5C).
For the activities of PPO in S. natans and U. vulgaris, significant increase in PPO activity was measured at Sinjak site in July (Fig. 6B).
In order to obtain a clearer insight into the relation between enzyme activities in selected plant species and environmental conditions at investigated sites, the analysis of Pierce's correlation coefficient was performed and the obtained results were presented in Tables 2 and 3. According to the large body of work, the value of Pierce's coefficient from 0.61 to 0.97 is considered a highly positive correlation and from −0.67 to −0.97 a highly negative correlation (Fitriansyah et al., 2017). Our results indicate significant correlation between solPOX activity in the root of P. communis and oxygen saturation at the Necik site. In the leaves, ionPOX activity in P. communis significantly correlated with air temperature at Necik site, and a correlation was also detected for roots and water temperature at the Sinjak site. Significant correlations between PPO activity in the underground tissues of P. communis (rhizome and root) and oxygen concentration at Sinjak site were observed. In addition, PPO activity in the root of P. communis also significantly correlated with pH at Sinjak site. In U. vulgaris, PPO activity was in significant positive correlation with water transparency as well as in significant negative correlation with electrical conductivity.
 |
Fig. 1 The change in the activities of solPOX (µmol−1mg prot −1 min−1) in the root (A), leaf (B) and rhizome (C) of P. communis at investigated sites Necik and Sinjak of Bardača wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
 |
Fig. 2 The change in the activities of ionPOX (µmol −1 mg prot −1 min−1) in the root (A), leaf (B) and rhizome (C) of P. communis at investigated sites Necik and Sinjak of Bardača wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
 |
Fig. 3 The change in the activities of solPOX (µmol −1 mg prot −1 min−1) (A), and ionPOX (B) of S. natans at investigated sites Necik and Sinjak of Bardača wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
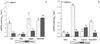 |
Fig. 4 The change in the activities of solPOX (µmol−1 ∙ mg prot −1 ∙ min−1) (A), and ionPOX (B) of U. vulgaris at investigated sites Necik and Sinjak of Barda?a wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
 |
Fig. 5 The change in the activities of PPO (µmol −1 mg prot −1 min−1) in the root (A), leaf (B) and rhizome (C) of P. communis at investigated sites Necik and Sinjak of Bardaca wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
 |
Fig. 6 The change in the activities of PPO (µmol −1∙ mg prot −1 ∙ min−1) in S. natans (A) and U. vulgaris (B) at investigated sites Necik and Sinjak of Bardaca wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
The values of Pierce's correlation coefficient between the enzyme activities of investigated plant species and physico-chemical parameters of water at Necik site* indicates that the correlation is significant at the p < 0.05 level.
The values of Pierce's correlation coefficient between the enzyme activities of investigated plant species and physico-chemical parameters of water at Sinjak site. * indicates that the correlation is significant at the p< 0.05 level.
4 Discussion
Decreased oxygen concentrations in the studied basins during the period (August–September), increase in temperature and light intensity, hypoxia, decreased transparency and increased turbidity, intensification of eutrophication were stress factors to which aquatic macrophytes were exposed during the investigated period (Tab. 1). Lower values in oxygen content (during spring–autumn) were probably the result of increased respiration and decomposition (Covich, 2001; Dalmacija et al., 2004; Dodds, 2006). Precipitation, agricultural activities during the autumn, early spring and winter months lead to an increase in electrical conductivity (Dalmacija et al., 2004), which was in accordance with the results obtained in this paper. Our results indicate that this area is characterized by a favourable oxygen regime, medium mineralization, mild alkalinity, increased turbidity, and reduced transparency at the end of the season and increased temperature, which is in line with previous research (Lolić, 2013; Maksimović et al., 2020b).
One of the first responses of plants to various abiotic and biotic stresses is an increase in POX activity. The activity of POX can vary depending on the stress factor as well as the length of its duration (Schröder et al., 2009; Dorantes et al., 2012; Alfadul and Al-Fredan, 2013), which is shown in our work. Changes in POX and PPO activity are known to be one of the plant's first responses to stress (Takahama and Oniki, 2000; Costantbel and Barbehenn, 2008; Dorantes et al., 2012 and Zuniga, 2012), so higher summer activities in all examined species at the studied sites are likely to be the result of the influence of changing physical and chemical conditions of the environment. Ferrees et al. (2011) showed that the higher activities of vacuole peroxidase were associated with the accumulation of H2O2, which occurred as a result of reduced photosynthesis especially in conditions of high temperatures and intense light. Increased activities of POX in our work during the summer may be associated with high light intensity and increased production of ROS (Figs. 1B, 3A and 5A). At the Sinjak site, solPOX activity in the leaves of Phragmites communis and Salvinia natans was higher than in Necik. The reason for this was probably the increased temperature at the Sinjak site, which could lead to the oxidative stress and induction of the investigated enzyme.
In P. communis leaves, lower ionPOX activity was measured compared to solPOX by 80% at the Necik site and by 77% at the Sinjak site. It is known that peroxidases bound to the cell wall participate in lignifications while soluble peroxidase isoforms function as “scavengers” of H2O2, so significantly higher activity in the soluble fraction is probably due to the influence of stress factors present during the seasons. The increase in ionPOX activity during maturation may be associated with increased cell wall lignification, indicating that there is a correlation between aging cell wall lignification and ionPOX activity, which is consistent with other studies (Takahama and Oniki, 2000; Hiraga et al., 2001; Almagro et al., 2009; Dorantes et al., 2012). Based on earlier data (Antonielli et al., 2002), we can assume that P. communis changes from C3 to C4 metabolism under conditions of increased temperature and light intensity, and as a result, defence mechanisms which induce increased activity of antioxidative enzymes are activated.
Significantly lower POX activities in soluble and ionic protein fractions at both investigated sites were measured in the root and rhizome of P. communis in relation to the leaves (Figs. 1 and 2), with higher root activity in relation to rhizome in both protein fractions. During the vegetation period, the activity of solPOX in the root and rhizome of P. communis changed periodically (Fig. X). Dorantes et al. (2012) suggested that in terrestrial plant species the roots were the first point of contact with various types of abiotic stress (drought, floods, heavy metals), and that the plants roots had higher POX activities compared to other organs. However, our results show the opposite, since the highest POX activities in the leaves were observed, probably due to different adaptive strategies of water macrophytes to the habitat conditions. Previous research showed that various phenolic compounds were substrates for peroxidases ionically bound to the cell wall, as well as to apoplastic peroxidases (Takahama, 2004). Lower peroxidase activity in the ionic fraction of POX of the studied species compared to soluble fractions may also indicate reduced availability of phenolic substrates.
Peroxidases are known to be involved in the catabolism of auxin, which is responsible for plant growth (Apel and Hirt, 2004; Passardi et al., 2005). Increased solPOX activity in Salvinia natans at the beginning of the season can be considered as one of the ways of participation of this enzyme in the management of plant growth processes (Fig. 3A). During the vegetation period, changes ionPOX activities in S. natans are probably the result of different environmental influences as well as morpho-anatomical characteristics of the species itself (Lizieri et al., 2012). Due to the fact that this is a floating plant that is constantly exposed to various stressors (high light, high temperatures, air pollutants, pathogens, herbicides), each of these factors can either directly or indirectly lead to excessive production of ROS and consequently affect activities of the POX and PPO, depending on the length of exposure.
This seasonal variability in the activities of the investigated enzymes can be related to changes in physico-chemical conditions of the aquatic environment (light, temperature, oxygen concentration) as well as specific morpho-anatomical characteristics of the tested species (absence of cuticle and mechanical elements, development of aerenchyma and the presence of bubbles). Different mechanical stimuli can induce changes in POX activity, as shown by Cipollini (1997). The authors stated that changes in POX activity could also be caused by the influence of wind, rain, water mass flow and the action of gravity on plant tissues. Given the fact that submerged plants are exposed to the water masses flow (Stevanović and Janković, 2001), it can be assumed that they are very often injured and that they are exposed to pathogens. Plant injuries can lead to induction of POX activity involved in lignification and cell wall stiffening (Almagro et al., 2009). This seasonal activity can be associated with phenolic compounds synthesis as well as the aging process (Kar and Mishra, 1976).
Steffens et al. (1994) showed that PPO activity increased in injured or damaged plant tissues in which cellular compartmentalization were lost. Changes in PPO activity depend on temperature and that the activity can be in the range of 20–80 °C. Ionită (2013) PPO is considered to be a defence enzyme in plants, so its increased activity period in June-July may be the response of plants to the influence of pathogens and mechanical damage (wind, mowers) (Vaughn and Duke, 1984; Constabel and Barbehenn, 2008). A research on wheat conducted by Altunkaya and Gökmen (2012) found that PPO activity increased to a certain limit along with growing temperature, and then decreased due to protein denaturation (already at 40 °C), which could be related to the results obtained in this paper. During the research period, a significant decline of PPO activity in S. natans, U. vulgaris and in the rhizome of P. communis in August and September was observed (Figs. 5C, 6A, 6B). These results are probably related to reduction of oxygen concentration in water during that period, since PPO is an enzyme that use oxygen as a substrate in the oxidation of phenolic compounds.
In the nutshell, these findings suggest that in P. communis, the activities of solPOX and PPO were the most sensitive to oxygen regime (in the underground tissues), while ionPOX activity was sensitive to air temperature (in the leaf). The correlation between PPO activity in U. vulgaris and electrical conductivity indicates sensitivity of this enzyme to decomposition of organic matter in Bardača wetland. The absence of significant correlations between the activities of all examined enzymes in S. natans and physico-chemical characteristics of water indicates better adaptation of this plant species to changes in environment. At the same time, the obtained oscillation of enzyme activities in S. natans could be the result of metabolic changes that occur during plant growth.
5 Conclusion
The results obtained in this paper indicate that changes in enzyme activities, in addition to abiotic stress, are also influenced by the plant species itself. Differences in POX and PPO activities were detected between emergent, submersed and floating plants, depending on both the season and site. The changes in the activities of solPOX, ionPOX and solPPO in examined water macrophytes can be used as biological indicators of environmental conditions.
Author contribution statement
Tanja Maksimovi? and Biljana Kukavica designed the research, conducted experiments, performed sampling, biochemical analyses, data analyses and manuscript writing. Dino Hasanagi? contributed to data analyses and manuscript writing. Ivan Samelak contributed to statistical analyses. All authors have read and approved the manuscript.
Conflict of interest
The authors declare they have no conflict of interest.
References
- Alfadul SMS, Al-Fredan MAA. 2013. Effects of Cd, Cu, Pb, and Zn combinations on Phragmites australis metabolism, metal accumulation and distribution. Arab J Sci Eng 38: 11–19. [CrossRef] [Google Scholar]
- Ali AA, Alqurainy F. 2006. Activities of antioxidants in plants under environmental stress, in The lutein-prevention and treatment for diseases , edited by N. Motohashi. Transworld Research Network, India, pp. 187–256. [Google Scholar]
- Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros-Barceló A, Pedernõ MA. 2009. Class III peroxidases in plant defence reactions. J Exp Bot 60: 377–390. [CrossRef] [PubMed] [Google Scholar]
- Altunkaya A, Gökmen V. 2012. Partial purification and characterization of polyphenoloxidase from durum wheat (Triticum durum L.). J Cereal Sci 55: 300–304. [CrossRef] [Google Scholar]
- Antonielli M, Pasqualini S, Batini P, Edreli L, Massacci A, Loreto F. 2002. Physiological and anatomical characterisation of Phragmites australis leaves. Aquat Bot 72: 55–66. [CrossRef] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399. [CrossRef] [PubMed] [Google Scholar]
- Chen KM, Gong HJ, Wang SM, Zhang CL. 2007. Antioxidant defence system in Phragmites communis Trin. ecotypes. Biol Plant 51: 754–758. [CrossRef] [Google Scholar]
- Cipollini Jr, DF. 1997. Wind-induced mechanical stimulation increases pest resistance in common bean. Oecologia 111: 84–90. [CrossRef] [PubMed] [Google Scholar]
- Constabel CP, Barbehenn R. 2008. Defensive roles of polyphenol oxidase in plants, in: Induced Plants Resistance to Herbivory, edited by A. Schaller. Netherlands: Springer, pp: 253–270. [CrossRef] [Google Scholar]
- Covich PA. 2001. Energy flow and ecosystems, in: Encyclopedia of Biodiversity , edited by S.A. Levin. San Diego, CA: Academic Press, pp. 509– 523. [CrossRef] [Google Scholar]
- Dalmacija B, Rončević S, Klašnja M, Krčmar D. 2004. Kvalitet voda, in Kontrola kvaliteta voda , edited by U. Dalmacija. Prirodno-matematički fakultet. Novi Sad: Institut za hemiju, pp. 15–32. [Google Scholar]
- Dodds WK. 2006. Eutrophication and trophic state in rivers and streams. Limnol Oceanogr 51: 671–680. [CrossRef] [Google Scholar]
- Dorantes AR, Angelica L, Zúñiga LAG. 2012. Phenoloxidases activity in root system and their importance in the phytoremediation of organic contaminants. J Environ Chem Ecotoxicol 4: 35–40. [Google Scholar]
- Đurić D, Sopić D, Trifković A, Jandrić B. 2004. Hidrotehnički radovi u području močvare Bardača, in: Život u močvari, Urbanistički zavod Republike Srpske, edited by Ž. Šarić, M. Stanković, D. Butler, a.d. Banja Luka, pp. 17–27. [Google Scholar]
- Ferreres F, Figueiredo R, Bettencourt S, et al. 2011. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? J Exp Bot 62: 2841–2854. [CrossRef] [PubMed] [Google Scholar]
- Fitriansyah SN, Fidrianny I, Ruslan K. 2017. Correlation of total phenolic, flavonoid and carotenoid content of Sesbania sesban (L. Merr) leaves extract with DPPH scavenging activities. Int J Pharm Pharmaceut Res 9: 89–94. [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. 2001. A large family of class III plant peroxidases. Plant Cell Physiol 42: 462–468. [CrossRef] [PubMed] [Google Scholar]
- Hossain MA, Piyatida P, da Silva JAT, Fujita M. 2012. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. Review Article. J Bot 10: 1–37. [Google Scholar]
- Ionită E. 2013. Plant polyphenol oxidases: isolation and characterization. Review Article. Innov Roman Food Biotechnol 13: 1–10. [Google Scholar]
- Kar M, Mishra D. 1976. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57: 315–319. [CrossRef] [PubMed] [Google Scholar]
- Kukavica BM., Veljović-Jovanović SD, Menckhoff L, Luthje S. 2012. Cell wall-bound cationic and anionic class III isoperoxidases of pea root: biochemical characterization and function in root growth. J Exp Bot 63: 4631–4645. [CrossRef] [PubMed] [Google Scholar]
- Lizieri C, Kuki KN, Aguiar R. 2012. The morphophysiological responses of free-floating aquatic macrophytes to a supra-optimal supply of manganese. Water Air Soil Pollut 223: 2807–2820. [CrossRef] [Google Scholar]
- Lolić S. 2013. Mikrobiološka analiza stanja kvaliteta vode ribnjaka Bardača-Doktorska disertacija. Univerzitet u Banjoj Luci. Prirodno-matematički fakultet, Studijski program Biologija. Banja Luka. [Google Scholar]
- Lowry OH, Rosebrough, NJ, Farr, AL, Randall, RJ. 1951. Protein measure?ment with the Folin phenol reagent. J Biol Chem 193: 265–275 [CrossRef] [PubMed] [Google Scholar]
- Maksimović T, Hasanagić D, Kukavica B. 2020a. Antioxidative response of water macrophytes to changes in the living environment during vegetation season: an experimental study, in: Environmental Concerns and Sustainable Development, edited by V. Shukla, N. Kumar. Chapter 6: Singapore: Springer Nature. [Google Scholar]
- Maksimović T, Lolić S, Kukavica B. 2020b. Seasonal changes in the content of photosynthetic pigments of dominant macrophytes in the Bardača fishpond area. Ekológia (Bratislava) 39: 201–213. [CrossRef] [Google Scholar]
- Mishra BB, Gautam S. 2016. Polyphenol oxidases: biochemical and molecular characterization, distribution, role and its control. Enzyme Eng 5: 1–9. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD). 1982. Eutrofication of Waters. Monitoring Assessment and Control. Paris. [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C. 2005. Peroxidases have more functions than a swiss army knife. Plant Cell Reports 24: 255–265. [CrossRef] [PubMed] [Google Scholar]
- Schröder P, Lyubenova L, Huber C. 2009. Uptake of heavy metals and their effects on the plant detoxification cascade in presence or absence of organic pollutants, in Proceedings of the 11tn International Conference on Environmental Science and Technology Chania, Crete, Greece, 3–5 September. [Google Scholar]
- Službeni glasnik Republike Srpske. 2001. Uredba o klasifikaciji voda i kategorizaciji vodotoka, br. 42. [Google Scholar]
- Steffens JC, Harel E, Hunt MD. 1994. Polyphenol oxidase, in Genetic Engineering of Plant Secondary Metabolism, edited by B.E. Ellis, G.W. Kuroki, H.A. Stafford. New York: Plenum Press, pp. 275–312. [CrossRef] [Google Scholar]
- Stevanović M, Janković MM. 2001. Ekologija biljaka sa osnovama fiziološke ekologije biljaka. Beograd, pp. 87–193, 261–275. [Google Scholar]
- Takahama U, Oniki T. 2000. Flavonoids and some other phenolics as substrates of peroxidase: physiological significance of the redox reactions. J Plant Res 113: 301–309. [CrossRef] [Google Scholar]
- Takahama U. 2004. Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: physiological significance of the oxidation reactions. Phytochem Rev 3: 207–219. [CrossRef] [Google Scholar]
- Teisseire H, Guy V. 2000. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci 153: 65–72. [CrossRef] [Google Scholar]
- Vaughn KC, Duke SO. 1984. Function of polyphenol oxidase in higher 575 plants. Physiolog Plant 60: 106–112. [CrossRef] [Google Scholar]
Cite this article as: Maksimović T, Hasanagić D, Samelak I, Kukavica B. 2022. Class III peroxidase and polyphenol oxidase activities in aquatic macrophytes during vegetative period in Bardača wetland. Int. J. Lim. 58: 8:
All Tables
Physico-chemical parameters of water at the investigated sites (Necik and Sinjak).
The values of Pierce's correlation coefficient between the enzyme activities of investigated plant species and physico-chemical parameters of water at Necik site* indicates that the correlation is significant at the p < 0.05 level.
The values of Pierce's correlation coefficient between the enzyme activities of investigated plant species and physico-chemical parameters of water at Sinjak site. * indicates that the correlation is significant at the p< 0.05 level.
All Figures
 |
Fig. 1 The change in the activities of solPOX (µmol−1mg prot −1 min−1) in the root (A), leaf (B) and rhizome (C) of P. communis at investigated sites Necik and Sinjak of Bardača wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
| In the text | |
 |
Fig. 2 The change in the activities of ionPOX (µmol −1 mg prot −1 min−1) in the root (A), leaf (B) and rhizome (C) of P. communis at investigated sites Necik and Sinjak of Bardača wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
| In the text | |
 |
Fig. 3 The change in the activities of solPOX (µmol −1 mg prot −1 min−1) (A), and ionPOX (B) of S. natans at investigated sites Necik and Sinjak of Bardača wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
| In the text | |
 |
Fig. 4 The change in the activities of solPOX (µmol−1 ∙ mg prot −1 ∙ min−1) (A), and ionPOX (B) of U. vulgaris at investigated sites Necik and Sinjak of Barda?a wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
| In the text | |
 |
Fig. 5 The change in the activities of PPO (µmol −1 mg prot −1 min−1) in the root (A), leaf (B) and rhizome (C) of P. communis at investigated sites Necik and Sinjak of Bardaca wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
| In the text | |
 |
Fig. 6 The change in the activities of PPO (µmol −1∙ mg prot −1 ∙ min−1) in S. natans (A) and U. vulgaris (B) at investigated sites Necik and Sinjak of Bardaca wetland. The values are expressed as mean of three repetition ± SE. Different small letters indicate statistical significance between months within the same site (a, b, c, d, e for Necik and a1, b1, c1, d1, e1, for Sinjak) according to LSD test (p < 0.05). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


