| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 24 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/limn/2021022 | |
| Published online | 29 October 2021 | |
Research Article
Spatial and temporal variation in species composition of ciliates communities (Alveolata, Ciliophora) from tropical urban and rural streams
1
Núcleo de Pesquisa em Limnologia, Ictiologia e Aquicultura, Programa de Pós-graduação em Ecologia de Ambientes Aquáticos Continentais, Universidade Estadual de Maringá - UEM, Av. Colombo, 5790, 87020-900 Maringá, PR, Brasil
2
Universidade Cesumar − UniCesumar. Instituto Cesumar de Ciência, Tecnologia e Inovação (ICETI)/Programa de Pós-graduação em Tecnologias Limpas: Sustentabilidade Ambiental. Av. Guedner, 1610, 87050-900 Maringá, PR, Brasil
3
Programa de Pós-graduação em Biologia Comparada, Universidade Estadual de Maringá, Av. Colombo, 5790, 87020-900 Maringá, Paraná, Brasil
4
Centro Universitário Ingá − UNINGÁ, Rod. PR 317, 6114 Parque Industrial 200, Maringá − PR, 87035-510 Maringá, Paraná, Brasil
* Corresponding author: fernando_toha@hotmail.com
Received:
29
June
2021
Accepted:
1
October
2021
The aim of this study was to investigate the spatial and temporal patterns in species composition of ciliates, in rural streams, affected by agricultural activities, and urban streams, impacted by domestic wastewater. Samplings were taken in two different periods of the year, in the headwater, middle and mouth stretch of ten streams. We recorded 143 species of ciliates, distributed in 14 groups, standing out Hymenostomatia, Peritrichia and Hypotrichia. Our results showed significant spatial (between rural and urban streams) and, especially, temporal differences (between winter and summer periods) in the ciliates taxonomic composition. Such differences seem to be not related to the organic load that was quite similar among streams and periods sampled. Rather, the changes in ciliates composition are probably driving mainly by other enviromental variables such as resources, determined by the spatial diferences in light availability, and flow water velocity and discharge, which present high temporal dissimilarity.
Key words: Tropical streams / lotic environmental / urban streams / protist
© EDP Sciences, 2021
1 Introduction
Anthropogenic activities implicate a series of environmental disturbances that directly affect aquatic ecosystem functioning (Segovia et al., 2016; Fañani et al., 2021). Rural and urban watersheds suffer from human activities, which alter the characteristics, the balance and the dynamics of natural resources hindering the supply of good quality water (Kuhl et al., 2010; Debastiani et al., 2016; Harfuch et al., 2019).
The increase in discharge of pollutant (be them pesticides or organic pollution) into water systems and urbanization, has often limited the multiple use of freshwater environments. Likewise, the ecosystem services provided by water bodies also become limited, given the impacts on these ecosystems (Madoni, 2005; Mandaric et al., 2018; Ullah et al., 2018).
These negative effects of urbanization on streams have been so prominent in recent years that Meyer et al. (2005) proposed the paradigm of the urban stream syndrome. This syndrome describes a set of symptoms common to lotic ecosystems, including changes in hydrology, increase in concentration of nutrients and contaminants, reduction in species richness with increased dominance of taxa tolerant to environmental disturbances (Walsh et al., 2005).
According Dias et al. (2008) physical and chemical characteristics, that have been traditionally used to evaluate the effect of these impacts, isolated from the analysis of the biotic community, do not provide enough evidence to completely evaluate water quality. Therefore, biological data associated with these environmental traits are essential tools to evaluate the water quality of rivers and streams and contribute to controlling the release of organic pollutants in urban lotic systems (Lippert et al., 2019; Martins et al., 2017).
Some studies have evidenced that the diversity of ciliates, in both the taxonomic (Bagatini et al., 2013; Dias et al., 2008; Madoni, 2005; Paiva and Silva-Neto, 2004), as well the functional diversity metric (Segovia et al., 2016; Meira et al., 2021), are useful bioindicators for monitoring water quality in tropical impacted streams, since they accurately reflect different anthropogenic disturbances. In addition, according to Camargo et al. (2012) it is essential to determine the major factors controlling the structure and dynamics of communities, to subsidize the elaboration of proposals for monitoring, management, conservation and restoration of these ecosystems, which have been extensively modified in the recent decades.
In tropical lotic environments, some protists are primarily controlled by seasonal variation in limnological features, determined by the rainfall regime (Camargo and Velho, 2011). On the other hand, spatially, the water quality, heavily affected by the human impacts from the land use and occupation, can be more important than the seasonality of the rainfall regime on the structure of these communities (Camargo et al., 2012). These authors found that, the water quality seems to be the most important factor for the structure of protists flagellates community composition in tropical streams under direct urban influence.
Although the ecological role of protist ciliates to the aquatic ecosystem metabolism has already been highlighted previously, this community is still little studied, mainly in tropical environments. In the same way, studies concerning ciliates from lotic environments are scarce throughout the world. Nevertheless, we must highlight the studies undertaken by Colzani and Alves (2013), Debastiani et al. (2016), Dias et al. (2008), Madoni (2005), Madoni and Braghiroli (2007), Mieczan et al. (2013, 2017), Rossi et al. (2016), Segovia et al. (2016) and Dias et al. (2021).
In this context, this study aimed to investigate the spatial and temporal flutuations in species composition of the protist ciliates community in10 urban and rural streams, in two distinct periods of the year (winter and summer). We predicted that: (i) distinct species composition will be found in rural and urban streams, considering the main source of impact of each type of stream (agricultural versus urban) and (ii) temporally, the species composition will change between the two periods of the year, considering the limnological distinctions drove by the temperature and rainfall regime.
2 Materials and methods
2.1 Study área
This study was undertaken using 10 streams within the urban and rural perimeter of Maringá County, Paraná State (Fig. 1), belonging to the Pirapó River watershed, which is found in the physiographic region of Third Plateau of the Paraná State (22°30′S and 23°30′S; 51°15′W and 52°15′W), with a catchment area of about 487.012 km2 (IBGE, 2021) that drains an area of approximately 5000 km2 (Segovia et al., 2016).
The climate is subtropical, with abundant rain during summer and dry winters, with mean annual temperatures above 20 °C. The average flow of the stream currents varies from 0.13 to 0.32 m/s (Segovia et al., 2016). The region is relatively industrialized and urbanized, and the Municipality of Maringá is the most important urban center in the region, having about 430,157 inhabitants (IBGE, 2020).
In several stretches of these streams there is intense siltation, due to the changes in riparian vegetation, which is caused mainly by anthropogenic activity and irregular use and occupation of the soil. Furthermore, there is wastewater input to most of the streams found in this urban area (personal observation).
Of the 10 studied streams, five streams were considered rural (Queçaba, Remo, Romeira, Roseira and Zaúna), and mainly affected by agricultural effluents, including fertilizers and pesticides (Fig. 1). The Guaiapó, Mandacaru, Miosótis, Nazaré and Pirapozinho streams were considered urban due to the impacts caused by domestic and industrial effluents. However, among these streams, only the Nazaré is totally within the urban area; the Mandacaru has its headwater and middle course in this area, whereas the rest of the streams have only headwater located in the urban perimeter (Fig. 1).
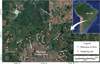 |
Fig. 1 Study area with the location of Pirapó River watershed and studied streams, Maringá County, Paraná State. |
2.2 Sampling and periodicity
Samplings of ciliates were performed during two periods of the year: during the winter of 2007 (August), and summer of 2008 (February). Subsurface samplings were taken in triplicate in three stretches of each stream, in the headwater, middle course and mouth, comprising 30 sampling sites and 180 samples.
For each sample, we collected two liters of water using plastic flasks. The samples were kept in thermal boxes until they arrived at the laboratory, where the analysis of species composition was undertaken.
In addition to biological communities, analyzes of limnologic variables were also measured: water temperature (°C), air temperature (°C), pH (Digimed potentiometer DM-23), conductivity (μS/cm; Digimed conductivimeter (DM-3P-E2), dissolved oxygen (mg/L; YSI 550A digital portable oximeter). Further samples were collected to determine the concentrations of total nitrogen (mg/L), phosphate (mg/L), the chemical demand of oxygen (mg O2/L), the biochemical demand of oxygen (mg O2/L) (APHA, 2012). For heavy metal analysis (Pb, Mo and Hg), the samples of water were kept refrigerated, and later sent to a specialized laboratory to quantify the measures and values of these attributes. The sample was centrifuging and after by inductively coupled plasma optical emission (ICP OES) for large and selected oligoelements. The spectrophotometry was done by atomic absorption according to American Public Health Association (APHA, 2012). For the determination of oils and fat, a solvent extraction method was used, according to Best and Ross (1977).
The current velocity measurements for each stream were made with the aid of a JDC Eletronic Flow-meter, model Flowatch (Cunico et al., 2012). The discharge was calculated using the equation: Q = A × V, where A = cross-sectional area of the channel, and V = the current velocity in m/s (Hauer and Lamberti, 2011). The canopy openness was quantified as a percentage of the water not covered by the natural spread of foliage from plants. At each sampling point, 25 measures were taken 10 cm apart using quadrants (0.50 m × 0.50 m), from visual observation to identify shading. The relative frequencies were calculated from the number of measures in which did not occur shading and the total number of measurements taken (Cunico et al., 2012, Segovia et al., 2016).
2.3 Laboratory analysis
The analysis of species composition of ciliates was performed in vivo, immediately after the samplings, to avoid the loss of cells and alterations in the cell's shape and dimensions. The taxonomic framework followed Adl et al. (2019).
Ciliate samples were concentrated to a volume of 100 mL, using a plankton net with a mesh size of 10 μm. Using monochannel pipettes, we analised 20 aliquots of 50 µL, totaling 1 mL for each sample. These aliquots were analyzed on glass slides under an optical microscope. With adequate magnifications (100×, 400× and 1000×), the ciliates were identified at specific levels whenever possible, using a specialized bibliography: Foissner et al. (1999), Foissner and Berger (1996) and Lynn (2008).
2.4 Data analysis
To characterize the studied environments, we summarized data from physical and chemical water variables in an Analysis of Principal Components (PCA). We used the Broken-Stick criterion (Jackson, 1993) to select the significant PCA axes. Before the analysis, environmental variables were log transformed, except for pH. PCA was performed using the function “prcomp” from vegan package (Oksanen et al., 2018).
The frequency of occurrence of each species (Dajoz constancy index-c) was calculated by the percentage of samples in which each species occurred. Based on their occurrence, the species were classified as constant (present in more than 50% of the samples), accessory (present in 25% to 50% of the samples) or accidental (present in less than 25% of the samples) (Dajoz, 1973).
For synthetize the temporal and spatial distribution of ciliates community, we performed an analysis of homogeneity of multivariate dispersions (PERMDISP; Anderson et al., 2006). This test is based on the average dissimilarities from each sample to the centroid of its group, in a multivariate space built using principal coordinate analysis (PCoA; Anderson et al., 2006). Thus, higher variations in community structure across sites (i.e., beta diversity) are depicted by greater dissimilarities to a group's centroid (Anderson et al., 2006). Four groups were considered, representing the combination of season and landscape type (summer rural (SR); summer urban (SU); winter rural (WR); winter urban (WU)). Statistical significance among group centroids was assessed through 999 permutations. Ordinations were performed on dissimilarity matrices generated using the Jaccard index, calculated from site-by-species presence-absence data, using the function “betadisper” from vegan package (Oksanen et al., 2018). In addition, we used Moran's I correlograms (Legendre and Legendre, 2012) to check if the control for spatial autocorrelation bias was required, which could somehow inflate the significance of each predictor.
Differences in the composition of the ciliate community between summer and winter seasons, as well as between rural and urban areas, were tested using a Permutational Multivariate Analysis of Variance (PERMANOVA; Anderson, 2005), applied to a species presence-absence matrix, using the function “adonis” from the R package vegan. The PERMANOVÁs assumption of homogeneity of dispersion among tested groups was verified with PERMDISP described above. The Jaccard distance was used as a measure of dissimilarity and 9999 permutations to assess the significance of the pseudo-F derived from PERMANOVA. Finally, to verify if differences in Jaccard dissimilarity are related to turnover or nestedness beta-diversity components (Baselga, 2010), we used the function “beta.multi” from the R package betapart.
3 Results
Results of the Principal Component Analysis (PCA) evidenced a higher temporal than spatial segregation of sampling units. In this way, the first PCA axis, the only significant (p < 0.05), discriminated samples from the winter period, negatively correlated to this axis, and characterized by the high values of dissolved oxygen, discharge and water flow, from the summer samples, positively correlated to this axis, and presenting higher values of air temperature, water temperature, BOD, DOC and PO4 (Fig. 2).The ordination also suggested, although not significantly (p > 0.05), a spatial segregation of the samples. In this way, most of samples from rural streams are negativily correlated to the second axis of PCA and characterized by the high values of canopy aperture, while urban streams were, in general, positively correlated with this axis and presented high nitrogen concentations, besides the highest values of conductivity, especially in the summer. More information about the physical and chemical characteristics of each stream sampled can be found in the supplementary material (Supplementary Material, Table S1).
We recorded 143 species of ciliates, belonging to 15 groups, among which Hymenostomatia (25 species), Peritrichia (15 species) and Hypotrichia (15 species) were the most representative (Tab. 1). In addition to these, which stood out in both types of streams, Pleurostomatida and Euplotida were also importante in urban streams and Prostomatea in rural ones (Fig. 3).
At the species level, when comparing the different types of streams, 116 species were reccorded in rural streams and 98 species in urban ones (Tab. 1).
Through the results from Dajoz index, we verified that Colpoda steinii Maupas, 1883, Glaucoma scintillans Ehrenberg, 1830, Urocentrum turbo (Mueller, 1786) Nitzsch, 1827, Aspidisca cicada (Mueller, 1786) Claparède and Lachmann, 1858, Vorticella convallaria Linnaeus, 1758, Coleps hirtus (Mueller, 1786) Nitzsch, 1827, Urotricha farcta Claparede and Lachmann, 1859 and Cinetochilum margaritaceum Perty, 1849 were the most constant species (Tab. 1). On the other hand, other species, as Actinobolina smalli Holt et al., 1973, Stentor multiformis (Mueller, 1786) Ehrenberg, 1838, Paramecium caudatum Ehrenberg, 1833, Stylonychia mytilus Ammermann, 1971, Opercularia coarctata (Claparède and Lachmann, 1858) Roux, 1901 and Urotricha furcata Schewiakoff, 1892 were found to be accidental (Tab. 1).
In rural streams, 40 exclusive species were recorded, such as Coleps elongatus Ehrenberg, 1831, Cothurnia annulata Stokes, 1885, Limnostrombidium sp., Loxodes magnus Stokes, 1887, Plagiocampa sp., Pseudomicrothorax dubius (Maupas, 1833) Penard, 1922, Rimostrombidium humile (Penard, 1922) Petz and Foissner (1992), Rimostrombidium lacustris (Foissner et al., 1988) Petz and Foissner, 1992, Sathrophilus muscorum (Kahl, 1931) Corliss, 1960 and Zoothamnium arbuscula (Ehrenberg, 1831) Ehrenberg, 1838 (Tab. 1). Otherwise, 29 species were registered only in urban streams, such as Amphileptus pleurosigma (Stokes, 1884) Foisnner, 1984, Drepanomonas revoluta Penard, 1922, Euplotes cf. aediculatus Pierson, 1943, Frontonia elliptica Beardsley, 1902, Opercularia coarctata, Oxytricha setigera Stokes (1891), Podophrya fixa (Mueller, 1786) Ehrenberg, 1833, Podophrya sp., Trochioides recta (Kahl, 1928) Kahl, 1931and Uronema nigricans (Mueller, 1786) Florentin, 1901 (Tab. 1).
The ordination based on PCoA axis reveal that higher dissimilarities in ciliates species composition occurred between the studied periods (August/winter and February/summer), although spatial segregation of samples had also been evidenced by the analysis (Fig. 4). In this way, the PERMANOVA evidencied significant differences in the species composition of ciliates community between periods (Pseudo F = 4.70; p < 0.001) and types of streams (Pseudo F = 2.69; p < 0.001). On the other hand, no pattern was observed for the stream stretches (Pseudo F = 0.94; p = 0.628). We found that total dissimilarity (0.981) was strongly related to turnover (0.973) than nestedness component (0.001).
PERMDISP results revealed that the magnitude of the dissimilarity was not significantly different among seasons and streams areas (p = 0.163) (Fig. 4). The spatial distribution of the sampled sites did not present bias related to spatial autocorrelation structures according to Moran's I correlograms (p = 0.137).
 |
Fig. 2 Principal Component Analysis (PCA) results based on the physical and chemical parameters measured in urban and rural streams, during two different hydrological periods (winter and summer). |
Faunistic survey and Dajoz index of ciliate protozoan community in rural and urban streams and respective regions (H = headwater, Mc = middle course, M = mouth), during two hydrological periods (dry and rainy) (+ = accidental, ++ = accessory, +++ = constant).
 |
Fig. 3 Relative composition of species number by order, for urban streams (A) and rural streams (B) in both summer and winter periods (Pir: Pirapozinho, Gua: Guaiapó, Mio: Miozótis, Naz: Nazaré, Man: Mandacaru, Que: Queçaba, Ros: Roseira, Zau: Zaúna, Rom: Romeira, Rem: Remo). |
 |
Fig. 4 Test for homogeneity of multivariate dispersions (PERMDISP) based on Principal Coordinates Analysis (PCoA) performed from data of species composition of planktonic ciliates in urban and rural streams, in both summer and winter periods. |
4 Discussion
The limnological characterization of the streams here investigated, derived from a PCA, evidenced greater environmental changes between the summer and winter samples, than between urban and rural streams. This heterogeneity between seasons was driven especially by temperature, as expected, but also by nutrients and organic matter (BOD and DOC), which showed high values in February (summer), and by O2 and hydrodynamic conditions (water flow and discharge), during the winter. Tropical streams present climatic variations related especially to the seasons, which influence the water regimes controlled by the rains, and which drive the physical and chemical struturation of these ecosystems (Amaral et al., 2015).
Spatially, we expected an expressive distinction between rural and urban streams, especially in terms of the type of impact (agro-industrial pollutants and organic load) and also vegetation cover. However, a weak difference was evidenced between these types of streams. The greater opening of canopy in rural streams is certainly associated with use of the soil for agricultural production (Allan and Castillo, 2007; Thompson and Townsend, 2004), while in the urban ones, at least part of the valley (and their vegetation) is protected by municipal law. On the other hand, the higher conductivity and pH in urban streams suggests an impact of industrial origin in these environments (Daniel et al., 2002; Silva et al., 2012).
In relation to the protist ciliates community, corroborating the trend recorded in other studies about ciliates from rivers and streams (Cleven, 2004; Madoni, 2005; Madoni and Braghiroli, 2007; Reiss and Schmid-Araya, 2008; Debastiani et al., 2016; Negreiros et al., 2017), Hymenostomatia, Hypotrichia and Peritrichia were the most representative in the present study. Most species of these groups are characterized as interstitial benthic; however, they are commonly found in pelagic zones from continental aquatic environments (Foissner et al., 1999; Cleven, 2004; Mansano et al., 2013; Negreiros et al., 2017; Pauleto et al., 2017; Abamo et al., 2020; Kaur et al., 2021). Among the species identified as constant for rural and urban streams, Cinetochilum margaritaceum, Urocentrum turbo, Coleps hirtus and Glaucoma scintillans have been considered by other authors to be the dominant taxa in ciliates river communities (Andrushchyshyn et al., 2007; Madoni and Braghiroli, 2007; Segovia et al., 2016).
Corroborating the pattern observed for the physical and chemical characterization of the studied streams, the results of ciliates species composition also showed a more remarkable between winter and summer than spatial change in the organization of the community. Such results evidenced that community of protist ciliates was mainly structured by the greater heterogeneity, responded to the changes in limnological and hydrodynamic conditions. In this way, species or even groups with more or less resistance to the mechanical action of the water flow (Kiss et al., 2009), must predominate in period with more or less water flow velocity.
The temporal influence of hydrodinamic has been registered for several aquatic stream communities (Lampert and Sommer, 1997; Fulone et al., 2008; Cavalheiro and Fialho, 2020). In this way, species or even groups with more or less resistance to the mechanical action of the water flow (Kiss et al., 2009), must predominate in period with more or less water flow velocity. Moreover, while periods with higher water flow and discharge may mitigate environmental impacts due to dilution of total dissolved solids (Ahearn et al., 2004). Periods with reduced water flow, the environments become more susceptible to point sources of pollution (Ramírez et al., 2014) increasing the concentration of nutrients, organic matter and pollutants (Hatt et al., 2004; Yang et al., 2020). Therefore, since the concentration of organic load and pollutants is an important factor influencing the distribution of organisms in streams and rivers (Segovia et al., 2016; Kaur et al., 2021), this may also have contributed to the variation of the Protist ciliates community between the summer and winter samples of the studied streams.
In respect to the spatial variation in species composition, the differences between rural and urban streams seem to be, especially related to the main food resource available in each one. In this way, in the rural streams, we found the riparian vegetation greatly reduced, resulting in greater availability of light. Under such conditions, the primary production is enhanced, favoring algivorous species (Segovia et al., 2016). On the other hand, in urban streams the high input of allochthonous organic matter (from the canopy and the industrial and domestic effluents), as well as the less light penetration due to greater canopy coverage, favor the growth of the bacterial community, and therefore, the predominance of bacterial ciliates (Segovia et al., 2016).
In this way, we observed the occurrence of several ciliates that are characteristically found in environments with a high input of organic matter, such as some streams from the urban zone (like Suctoria). On the other hand, in some rural areas, we found species typical of environments with low organic loading (e.g., species from the Oligotrichea). Moreover, the replacement of Prostomatea in rural streams by Pleurostomatida in urban ones, suggest an advantage for creeper species in these environments.
In turn, no environmental heterogeneity among stretches (headwater, middle course or mouth) was evidenced by PCA, and this low variability was also expressed by the ciliates community, at least in terms of composition. Thus, any longitudinal variation in limnological conditions, if there is any, seems to be of little importance in driving the organization of ciliate communities, at least in the studied streams.
Considering the components of beta diversity envolved in changing species composition, we found that total dissimilarity of ciliates was explained by turnover component. Using a similar approach to partition beta diversity into the turnover and nestedness components (Baselga, 2010), Soininen et al. (2018) observed that the turnover component was clearly more important that the nestedness component in a meta-analysis of 269 data points.
In synthesis, our results showed significant differences in the composition of ciliate communities between the types of streams (rural and urban) but, especially, between periods of the year (winter and summer). Such spatial and temporal differences in the ciliates community structure and dynamics are probably driving by the resources, determined by the spatial diferences in light availability, and flow water velocity and discharge, which present high dissimilarity among studied periods.
Author contributions
L.F.M.V. and F.A.L.T idealized and coordinated the research. S.F.R.C, G.M.A., F.M.L.T., and L.F.M.V, participated in the samplings, laboratorial and data analysis; B.R.M., G.M.A., S.F.R.C, F.M.L.T., F.A.L.T., F.R.O and L.F.M.V participated in the writing and revision of the manuscript.
Supplementary Material
Supplementary file supplied by the authors. Access here
Acknowledgements
We thank to CNPq (Project “Identificação de potenciais bioindicadores em ecossistemas aquáticos urbanos: resposta de três grupos de organismos a gradientes de estresse”) and Capes/CNPq for scholarships. We are also thanking to Nupélia/PEA/PGB/UEM by the financial and logistic support. The researchers developed by LFMV and FALT have been continuously supported by CNPq.
References
- Abamo F, Cosain H, Abato J, Disoma C. 2020. Seasonal and Spatial Variation of Ciliate Abundance in Lake Lanao along Wato-Balindong, Lanao Del Sur, Philippines. IOP Conf Ser Earth Environ Sci 528: 012034. [CrossRef] [Google Scholar]
- Adl SM, Bass D, Lane CE, Lukeš J, Schoch CL, Smirnov A, et al. 2019. Revision to the classification, nomenclature, and diversity of Eukaryotes. J Euk Microbiol 66: 4–119. [CrossRef] [PubMed] [Google Scholar]
- Ahearn DS, Sheidley RW, Dahlgren RA, Keller KE. 2004. Temporal dynamics os stream water chemistry in the last free- flowing river draining the western Sierra Nevada, California. J Hydrol 293: 47–63. [CrossRef] [Google Scholar]
- Allan JD, Castillo MM. 2007. Stream ecology: structure and function of running waters. London: Chapman & Hall, 436 p. [Google Scholar]
- Amaral PHM, Silveira LS, Rosa BFJVR, Oliveira VC, Alves RG. 2015. Influence of habitat and land use on the assemblages of Ephemeroptera, Plecoptera, and Trichoptera in neotropical streams. J Insect Sci 15: 1–7. [CrossRef] [PubMed] [Google Scholar]
- Anderson MJ, Ellingsen KE, Mc Ardle BH. 2006. Multivariate as a measure of beta diversity. Ecol Lett 9: 683–693. [CrossRef] [PubMed] [Google Scholar]
- Anderson MJ. 2005. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. Department of Statistics, University of Auckland, New Zealand. Ecol Monogr 83: 557–574. [Google Scholar]
- Andrushchyshyn OP, Wilson PK, Williams DD. 2007. Ciliate communities in shallow groundwater: seasonal and spatial characteristics. Freshw Biol 52: 1745–1761. [CrossRef] [Google Scholar]
- APHA. 2012. Standard Methods for the Examination of Water and Wastewater. 22nd ed. Washington, DC: American Public Health Association, American Water Works Association and Water Environmental Federation, 1360 p. [Google Scholar]
- Bagatini IL, Spínola ALG, Peres BM, et al. 2013. Protozooplankton and its relationship with environmental conditions in 13 water bodies of the Mogi-Guaçu basin, SP, Brazil. Biota Neotrop 13: 152–163. [CrossRef] [Google Scholar]
- Baselga A. 2010. Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19: 134–143 [Google Scholar]
- Best GA, Ross SL. 1977. River pollution studies. Liverpool: Liverpool University Press, 92 p. [Google Scholar]
- Camargo JC, Velho LFM. 2011. Longitudinal variation of attributes from flagellate protozoan community in tropical streams. Acta Sci Biol Sci 33: 161–169. [CrossRef] [Google Scholar]
- Camargo JC, Vieira LCG, Velho LFM. 2012. The role of limnological variables and habitat complexity in impacted tropical streams as regulatory factors on the flagellate protozoa community. Acta Limnol Bras 24: 193–206. [CrossRef] [Google Scholar]
- Cavalheiro LW, Fialho CB. 2020. Fishes community composition and patterns o species distribution in Neotropical streams. Biota Neotrop 20: e20190828. [CrossRef] [Google Scholar]
- Cleven EJ. 2004. Seasonal and spatial distribution of ciliates in the sandy hyporheic zone of a lowland streams. Eur J Protistol 40: 71–84. [CrossRef] [Google Scholar]
- Colzani E, Alves MAM. 2013. Riqueza e distribuição de eucariontes unicelulares em três córregos sob influência antrópica na cidade de Ivinhema, Mato Grosso do Sul, Brasil. Rev Ambient Água 8: 192–203. [Google Scholar]
- Cunico AM, Ferreira EA, Agostinho AA, Beaumord AC, Fernandes R. 2012. The effects of local and regional environmental factors on the structure of fish assemblages in the Pirapó Basin, Southern Brazil. Landsc Urban Plan 105: 336–344. [CrossRef] [Google Scholar]
- Dajoz R. 1973. Ecologia geral. Vozes, Petrópolis, 472 p. [Google Scholar]
- Daniel MHB, Montebelo BA, Bernardes MC, et al. 2002. Effects of urban sewage on dissolved oxygen dissolved inorganic and organic carbon, and electrical conductivity of small streams along a gradient of urbanization in the Piracicaba River basin. Water Air Soil Pollut 136: 189–206. [CrossRef] [Google Scholar]
- Debastiani C, Meira BR, Lansac-Tôha FM, Velho LFM, Lansac-Tôha FA. 2016. Protozoa ciliates community structure in urban streams and their environmental use as indicators. Br J Biol 76: 1043–1053. [CrossRef] [PubMed] [Google Scholar]
- Dias RJP, Wieloch AH, D'Agosto M. 2008. The influence of environmental characteristics on the distribution of ciliates (Protozoa, Ciliophora) in an urban stream of southeast Brazil. Braz J Biol 68: 287–295. [CrossRef] [PubMed] [Google Scholar]
- Dias RJP, Souza PM, Rossi MF, Wieloch AH, Silva-Neto ID, D'Agosto M. 2021. Ciliates as bioindicators of water quality: A case study in the neotropical region and evidence of phylogenetic signals (18S-rDNA). Environ Pollut 268: 115760. [CrossRef] [PubMed] [Google Scholar]
- Fañani AB, Cibils-Martina L, Casset MA, Banegas BP, Poretti TI, Rocha L. 2021. Specific indicator invertebrates of urbanized habitats in tributary streams of the Luján River basin (Buenos Aires, Argentina). Ann Limnol Int J Lim 57: 12. [CrossRef] [EDP Sciences] [Google Scholar]
- Foissner W, Berger HA. 1996. User-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes and waste waters, with notes on their ecology. Freshw Biol 35: 375–482. [CrossRef] [Google Scholar]
- Foissner W, Berger H, Schaumburg J. 1999. Identification and ecology of limnetic plankton ciliates. Munich: Bavarian State Office for Water Management, 793 p. [Google Scholar]
- Fulone LJ, Vieira LCG, Velho LFM, Lima AF. 2008. Influence of depth and rainfall on testate amoebae (Protozoa-Rhizopoda) composition from two streams in northwestern São Paulo State. Acta Limnol Bras 20: 29–34. [Google Scholar]
- Harfuch CAC, Oliveira FR, Meira BR., et al. 2019. Qualidade da água no trecho superior da bacia do rio Pirapó: um rio urbano no sul do brasil. R Gest Sust Ambient 8: 513–538. [CrossRef] [Google Scholar]
- Hatt BE, Fletcher TD, Christopher JW, Taylor SL. 2004. The influence of urban density and drainage infrastructure on the concentration and loads of pollutants in small streams. Environ Manag 34: 112–124. [Google Scholar]
- Hauer FR, Lamberti G. (Eds.). 2011. Methods in stream ecology. Academic Press. [Google Scholar]
- IBGE, Instituto Brasileiro de Geografia e Estatística. 2020. Diario oficial da União Portaria n° PR-254, de 25 de agosto de 2020. População residente segundo as unidades da federação e municípios. Available in: https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=27/08/2020andjornal=515andpagina=71andtotalArquivos=195. Access: 21 jun. 2021. [Google Scholar]
- IBGE, Instituto Brasileiro de Geografia e Estatística. 2021. Cidades e estados. Available in: https://www.ibge.gov.br/cidades-e-estados/pr/maringa.html. Access: 21 jun. 2021. [Google Scholar]
- Jackson D. 1993. Sampling rules in principal componentes analysis: a comparison of heuristical and statistical approaches. Ecology 74: 2204–2214. [CrossRef] [Google Scholar]
- Kaur H, Warren A, Kamra K. 2021. Spatial variation in ciliate communities with respect to water quality in the Delhi NCR stretch of River Yamuna, India. Eur J Protistol 79: 125793. [CrossRef] [PubMed] [Google Scholar]
- Kiss ÁK, Ács É, Kiss KT, Török JK. 2009. Structure and seasonal dynamics of the protozoan community (heterotrophic flagellates, ciliates, amoeboid protozoa) in the plankton of a large river (River Danube, Hungary). Eur J Protistol 45: 121–138. [CrossRef] [PubMed] [Google Scholar]
- Kuhl AM, Rocha CLMSC, Espíndola ELG, Lansac-Toha FA. 2010. Rural and urban streams anthropogenic influences and impacts on water and sediment quality. Int Rev Hydrobiol 95: 260–272. [CrossRef] [Google Scholar]
- Lampert W, Sommer U. 1997. Limnology: the ecology of lake and streams. Oxford: University Press, 382 p. [Google Scholar]
- Legendre P, Legendre LF. 2012. Numerical Ecology. Amsterdam: Elsevier, 990 p. [Google Scholar]
- Lippert MAM, Lansac-Toha FM, Meira BR, Velho LFM, Lansac-Toha FA. 2019. Structure and dynamics of the protoplankton community in an environmentally protected urban stream. Braz J Biol 80: 844–859. [Google Scholar]
- Lynn D. 2008. The ciliated Protozoa: characterization, classification, and guide to the literature. Heidelberg: Springer, 128 p. [Google Scholar]
- Madoni P. 2005. Ciliated protozoan communities and saprobic evaluation of water quality in the hilly zone of some tributaries of the Po River (Northern Italy). Hydrobiologia 541: 55–69. [CrossRef] [Google Scholar]
- Madoni P, Braghiroli S. 2007. Changes in the ciliate assemblage along a fluvial system related to physical, chemical and geomorphological characteristics. Eur J Protistol 43: 67–75. [CrossRef] [PubMed] [Google Scholar]
- Mandaric L, René-Mor J, Sabater S, Petrovic M. 2018. Impacto of urban chemical pollution on water quality in small, rural and efluente-dominated Mediterranean streams and rivers. Sci Total Environ 613–614: 763–772. [CrossRef] [PubMed] [Google Scholar]
- Mansano AS, Hisatugo KF, Leite MA, Luzia AP, Regali-Seleghim MH. 2013. Seasonal variation of the protozooplanktonic community in a tropical oligotrophic environment (Ilha Solteira reservoir, Brazil). Braz J Biol 73: 321–330. [CrossRef] [PubMed] [Google Scholar]
- Martins RT, Couceiro SEM, Melo AS, Moreno MP, Hamada N. 2017. Effects of urbanization on stream benthic invertebrate communities in Central Amazon. Ecol Indic 73: 480–491. [CrossRef] [Google Scholar]
- Meira BR, Progênio M, Corrêa Leite E, et al. 2021. Functional feeding groups of Protist Ciliates (Protist: Ciliophora) on a neotropical flood plain. Ann Limnol Int J Lim (In press). [Google Scholar]
- Meyer JL, Paul MJ, Taulbee WK. 2005. Stream ecosystem function in urbanizing landscapes. J N Am Benthol Soc 24: 602–612. [CrossRef] [Google Scholar]
- Mieczan T, Adamczuk M, Tarkowska-Kukuryk M. 2017. Ecology of ciliates in microbial mats in meltwater streams, King George Island, maritime Antarctica. Polar Biol 40: 1071–1083. [CrossRef] [Google Scholar]
- Mieczan T, Górniak D, Świątecki A, Zdanowski M, Tarkowska-Kukuryk M. 2013. The distribution of ciliates on Ecology Glacier (King George Island, Antarctica): relationships between species assemblages and environmental parameters. Polar Biol 36: 249–258. [CrossRef] [Google Scholar]
- Negreiros OP, Segovia BT, Lansac-Tôha FM, et al. 2017. Structure and dynamic of planktonic ciliate community in a large neotropical river: the relevance of the pluviosity and tributaries in the biodiversity maintenance. Acta Limnol Bras 29: e101. [CrossRef] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, et al. 2018. Vegan: Community Ecology Package. Ordination methods, diversity analysis and other functions for community and vegetation ecologists. Version 2.5-1. Available in: https://cran.r-project.org/web/packages/vegan/index.html. Access: 22 jun. 2021. [Google Scholar]
- Paiva TDS, Silva-Neto ID. 2004. Ciliate protists from Cabiúnas Lagoon (Restinga de Jurubatiba, Macaé, Rio de Janeiro) with emphasis on water quality indicator species and description of Oxytricha marcili sp. n. Braz. J Biol 64: 465–478. [Google Scholar]
- Pauleto GM, Oliveira FR, Segovia BT, et al. 2017. Intra-annual variation in planktonic ciliate species composition (Protista: Ciliophora) in different strata in a shallow floodplain lake. Acta Limnol Bras 29: e107. [CrossRef] [Google Scholar]
- Ramírez A, Rosas KG, Lugo AE, Ramos-González OM. 2014. Spatio-temporal variation in stream water chemistry in a tropical urban watershed. Ecol Soc 19: 45. [CrossRef] [Google Scholar]
- Reiss J, Schmid-Araya JM. 2008. Existing in plenty: abundance, biomass and diversity of ciliates and meiofauna in small streams. Freshw Biol 53: 652–668. [CrossRef] [Google Scholar]
- Rossi A, Boscaro V, Carducci D, et al. 2016. Ciliate communities and hidden biodiversity in freshwater biotopes of the Pistoia province (Toscana, Italy). Eur J Protistol 53: 11–19. [CrossRef] [PubMed] [Google Scholar]
- Segovia BT, Lansac-Toha FM, Meira BR, Cabral AF, Lansac-Tôha FA, Velho LFM. 2016. Anthropogenic disturbances influencing ciliate functional feeding groups in impacted tropical streams. Environ Sci Pollut Res 23: 20003–20016. [CrossRef] [PubMed] [Google Scholar]
- Silva DML, Camargo PB, McDowell WH, Vieira I, Salomão MSB, Martinelli LA. 2012. Influence of land use changes on water chemistry in streams in the State of São Paulo, southeast Brazil. An Acad Bras Cienc 84: 919–930. [CrossRef] [PubMed] [Google Scholar]
- Soininen J, Heino J, Wang J. 2018. A meta‐analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob Ecol Biogeogr 27: 96–109. [CrossRef] [Google Scholar]
- Thompson RM, Townsend CR. 2004. Land-use influences on New Zealand stream communities: effects on species composition, functional organisation, and food-web structure. New Zeal J Mar Fresh Res 38: 595–608. [CrossRef] [Google Scholar]
- Ullah KA, Jiang J, Wang P. 2018. Land use impacts on surfasse water quality by statistical approaches. Glob J Environ Sci Manag 4: 231–250. [Google Scholar]
- Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP. 2005. The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24: 706–723. [CrossRef] [Google Scholar]
- Yang L, Yang G, Li H, Yuan S. 2020. Effects of rainfall intensities on sediment loss and phosphorus enrichment ratio from typical land use type in Taihu Basin, China. Environ Sci Pollut Res 27: 12866–12873. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Velho LFM, de Castro SdFR, Lansac-Tôha FM, Meira BR, de Oliveira FR, Alves GM, Lansac-Tôha FA. 2021. Spatial and temporal variation in species composition of ciliates communities (Alveolata, Ciliophora) from tropical urban and rural streams. Ann. Limnol. - Int. J. Lim. 57: 24
All Tables
Faunistic survey and Dajoz index of ciliate protozoan community in rural and urban streams and respective regions (H = headwater, Mc = middle course, M = mouth), during two hydrological periods (dry and rainy) (+ = accidental, ++ = accessory, +++ = constant).
All Figures
 |
Fig. 1 Study area with the location of Pirapó River watershed and studied streams, Maringá County, Paraná State. |
| In the text | |
 |
Fig. 2 Principal Component Analysis (PCA) results based on the physical and chemical parameters measured in urban and rural streams, during two different hydrological periods (winter and summer). |
| In the text | |
 |
Fig. 3 Relative composition of species number by order, for urban streams (A) and rural streams (B) in both summer and winter periods (Pir: Pirapozinho, Gua: Guaiapó, Mio: Miozótis, Naz: Nazaré, Man: Mandacaru, Que: Queçaba, Ros: Roseira, Zau: Zaúna, Rom: Romeira, Rem: Remo). |
| In the text | |
 |
Fig. 4 Test for homogeneity of multivariate dispersions (PERMDISP) based on Principal Coordinates Analysis (PCoA) performed from data of species composition of planktonic ciliates in urban and rural streams, in both summer and winter periods. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


