| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 18 | |
| Number of page(s) | 12 | |
| DOI | https://doi.org/10.1051/limn/2021016 | |
| Published online | 28 September 2021 | |
Research Article
Survival strategies of phytoplankton functional groups to environmental factors in a drinking water reservoir, central China
1
Guiyang University, Guiyang 550005, PR China
2
College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, PR China
3
State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, PR China
4
University of Chinese Academy of Sciences, Beijing 100049, PR China
5
China Water Environment Group Limited, Beijing 101101, PR China
6
Changsha Agricultural Comprehensive Administrative Law Enforcement Bureau, Changsha 410013, PR China
* Corresponding authors: wangxiao8258@126.com; yushunchen@ihb.ac.cn
Received:
19
September
2020
Accepted:
1
September
2021
In this study, use survival strategies of phytoplankton functional groups to environmental factors in a drinking water reservoir. Survival strategies of phytoplankton in drinking water reservoirs were rarely analysed. Dynamics and survival strategies of phytoplankton community in Zhushuqiao Reservoir (Changsha, China) were studied bimonthly from April 2016 to February 2017 to fill this gap. In spring, species of CRS-strategy that adapted to low water temperature, light, and nutrient dominated. There were small individuals of opportunistic colonists of C-strategy observed before stratification. With the increase of nutrient and water temperature in summer, slightly bigger, disturbance-tolerant species of R-strategy and species of CS-strategy that adapted to stratification dominated. In winter, some species adapted to low water temperature, which were R-strategists. Key factors driven seasonal phytoplankton succession were water temperature, total phosphorus, and dissolved inorganic nitrogen. Attention should be paid to potential threats from algal bloom species with C-strategy, and future longer-term monitoring of the system and its surrounding watersheds is greatly needed.
Key words: Phytoplankton functional groups / C-S-R survival strategies / environmental factors / reservoir / drinking water supply
© EDP Sciences, 2021
1 Introduction
Phytoplankton in aquatic ecosystems is crucial as it accounts for half of the primary production of the earth (Arrigo, 2005; Kruk and Segura, 2012). Phytoplankton species in different aquatic ecosystems may have similar ecological roles, and show similar seasonal responses to environmental changes (Salmaso et al., 2014). Biomass and composition of phytoplankton species are important components that can affect phytoplankton community structures (Sandgren, 1988, Santos and Calijuri, 1998). Phytoplankton community structures in aquatic ecosystems are consequences of interactions between the life cycle of species and natural selections (Alvesdesouza et al., 2008).
An approach to studying phytoplankton communities is by defining phytoplankton functional groups, which are based on phytoplankton ecological characteristics and growth strategies. Functional groups include species that have similar physiology, morphology, and ecology, and frequently coexist. The functional groups were based on morphological, physiological, and ecological attributes similarities of species, and a total of 38 functional groups were classified with different codes (Reynolds et al., 2002; Padisák et al., 2009). Moreover, the concept of functional groups has been widely applied to both freshwater (Hambright and Zohary, 2000; Kruk et al., 2002; Alvesdesouza et al., 2006, 2008; Hu et al., 2015) and marine phytoplankton (Smayda and Reynolds, 2001, 2003; Crossetti and Bicudo, 2008) studies.
Phytoplankton r and K selections have been reported in many studies, including Margalef (1978), Sommer (1981), Carney and Goldman (1988), and Arauzo and Cobelas (1994). Based on the r and K selection for terrestrial plants (Grime, 1979) and functional groups were classified, Reynolds et al. (2002) proposed a classification approach of C-R-S strategists for freshwater phytoplankton. The functional groups were classified first and then the C-R-S growth strategies were classified for each functional group. C-R-S strategists considered functional groups' sensitivity, tolerance and utilization of limited resources (Reynolds et al., 2002; Padisák et al., 2009; Salmaso et al., 2014) and main environmental constraints. The C-R-S growth strategies are as follows: C-strategists refer to competitive species with both low intensity of disturbance and low grazing stress. S-strategists are stress-tolerant species, and they adapt to low disturbance but high stress. R-strategists are disturbance-tolerant species, which are dominant in lakes with high disturbance but low stress (Reynolds, 1988, 1996).
Based on the difference of functional traits, phytoplankton species, assemblages, and communities are classified according to their different responses to environmental conditions, including their resource-obtaining methods and survival strategies under stress (e.g., precipitation and grazing) (Margalef, 1978; Scheffer et al., 1997, 2003; Naselli-Flores et al., 2007). Therefore, habitat templates and growth strategies for different functional groups could be established through their traits and environment (Reynolds et al., 2002; Salmaso and Padisák, 2007).
Most of previous studies were conducted in shallower reservoirs (e.g., Crossetti and Bicudo, 2008; Hu et al., 2015) or marine systems (e.g., Smayda and Reynolds, 2003; Alvesdesouza et al., 2008) while few focusing on spatial and temporal variations of phytoplankton growth strategies in deep drinking water reservoirs. Partly because of shallower water bodies, most previous studies did not consider different layers of the entire water column (e.g., Arauzo and Cobelas, 1994; Santos and Calijuri, 1998; Hu et al., 2015). In addition to seasonal succession of dynamic changes of phytoplankton communities, vertical distributions of phytoplankton and environmental factors in water are closely related to morphological and physiological properties of phytoplankton, and their survival strategies (Reynolds, 1987; Santos and Calijuri, 1998). Different phytoplankton species sharing similar morphological and physiological adaptations also share similar ecological growth and survival strategies (Reynolds, 1987; Smayda and Reynolds, 2003; Salmaso et al., 2014). In this study, we studied phytoplankton survival strategies and environmental conditions in the Zhushuqiao reservoir to (1) detect Spatial and temporal dynamics characteristics of phytoplankton survival strategies (C-S-R), (2) we expected variability in survival strategies (C-S-R) of phytoplankton to be driven by the physical factors, and (3) assess quantitative relationships between phytoplankton survival strategies and environment, which may help for future reservoir management.
2 Materials and methods
2.1 Study site
Zhushuqiao reservoir is one of the main sources of drinking water for the city of Changsha, capital of Hunan province, central China. It was developed from damming a tributary of the Xiang River (27.5°–28.5° N, 113°−115° E) in Liuyang city (Fig. 1). It has a watershed area of 564 km2 and a volume of 2.7 × 108 m3. The reservoir has a mean depth of 25 m (Maximum depth of 65 m). The local climate is subtropical monsoon, seasonal changes are very pronounced, with an annual mean air temperature of 17.5 °C and annual average precipitation of 1601mm, Yang (2008).
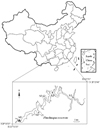 |
Fig. 1 Location of Zhushuqiao Reservoir and the sampling sites. |
2.2 Sampling of abiotic and biotic variables
A total of five sampling sites were set up from upstream to downstream along the Zhushuqiao reservoir. Stratified water sampling for abiotic and biotic variables was conducted bimonthly in the entire water column from the surface at 0.5 m layer to the bottom, from April 2016 to February 2017. The water depth was around 12 m in sites 1 and 2, which were sampled at an interval of 3 m. The water depths were around 30 m, 40 m and 50 m in sites 3, 4 and 5, respectively, and these sites were sampled at an interval of 5 m.
The phytoplankton sampling was determined using specifications for freshwater plankton surveys SC/T 9402–2010 in china. Qualitative phytoplankton samples were collected by passing through a #25 plankton net (pore size = 0.064 mm) into 100 ml brown plastic sampling bottles. Quantitative phytoplankton samples were collected by a cylindrical sampler and filled into 1L brown plastic sampling bottles. All collected samples were fixed with formalin (3–5%) and Lugol's solution in the field. In the laboratory, the quantitative samples were settled 48–72 h, and the supernatant from each sample was siphoned off and the residual was concentrated into 50 ml after sedimentation. After a thorough mixing of the 50 ml concentrated sample, 0.1 ml of the sample was identified and counted in a phytoplankton counting chamber under a direct microscope (Nikon E100) by following Hu and Wei (2006). The phytoplankton was counted by the random field method where at least 400 units in each sample were counted. Phytoplankton biomass was calculated using phytoplankton density and biovolumes (Sun et al., 1999; Hillebrand et al., 1999). Phytoplankton C-R-S strategists were assessed by following Reynolds (1997). Phytoplankton functional groups were following Reynolds et al. (2002) and Padisák et al. (2009) (Tab. 1).
Water temperature (WT, °C), dissolved oxygen (DO, mg L−1), and pH were measured in situ using a portable YSI (6600V2) probe. Illumination intensity (II) was measured by an illuminometer under water. Water transparency depth (SD, m) was measured using a Secchi disk. Laboratory analysis of physical and chemical variables of water was performed following standard methods for water quality analysis GB3838-2002, including total nitrogen (TN, mg L−1), ammonium (NH4-N, mg L−1), nitrite (NO2-N, mg L−1), nitrate (NO3-N, mg L−1), total phosphorus (TP, mg L−1), dissolved inorganic phosphorus (PO4-P, mg L−1) and chlorophyll a (Chl-a, mg L−1).
Phytoplankton functional groups and their growth strategies.
2.3 Phytoplankton data and statistical analyses
The relationships between environmental variables and the phytoplankton functional groups were tested using Pearson's correlation in R by vegan package. RDA (redundancy analysis) of the relationship between environmental factors and phytoplankton C-R-S strategies in Zhushuqiao reservoir was conducted in CANOCO 4.5. A detrended correspondence analysis (DCA) was first run to test whether the gradient fitted in linear, which directed us to use a redundancy analysis (RDA). The RDA was conducted to detect relationships between environmental factors and phytoplankton survival strategies in Zhushuqiao reservoir, to determine the main environmental factors affecting phytoplankton survival strategies variations. For the RDA, the data of environmental factors (except pH) and phytoplankton C-R-S strategies were log(x + 1)-transformed to reduce skewness, the RDA was tested using a Monte Carlo permutation test and considering a significant P-level of 0.05.
Finally, we used Nonmetric Multidimensional Scaling (NMDS) to analyze the spatial and temporal dynamics of phytoplankton assemblages' dissimilarity in different sites (Bray and Curtis, 1957; Salmaso, 1996). A total of 7 phytoplankton survival strategies included in the analysis. The Bray-Curtis dissimilarity was used as the distance metric. Stress values were used to measure the goodness of fit of NMDS analysis results: stress <0.05 is excellent, stress <0.1 is good, stress <0.2 is common, and stress >0.3 is poor and the NMDS analysis should consider adding the number of ordination axes (Kruskal, 1964). This was computed on a two-way permutational multivariate analysis of variance in R by the vegan package.
3 Results
3.1 Abiotic variables
Descriptive analyses of abiotic variables are given in Table 2, Figures 2 and 3. Regarding temperature and Illumination intensity followed the seasonal distribution, while pH did not show any seasonal tendency (Tab. 2). The five sampling stations in Zhushuqiao reservoir were considered significantly different only SD (p < 0.05). The water temperature ranged from 12.6 °C to 33.7 °C, with an average of 21.2 °C. Both water temperature and illumination intensity decreased from surface to bottom. Surface water temperature exceeded 25 °C from June to October. There was thermal stratification in water (reaching 25 m depth) from April to August (Fig. 2). SD ranged from 1.6 to 2.6 m (average of 2.04 m), the higher value was recorded in August and October. pH ranged from 6.8 to 8.2 (average of 7.5), the higher values were recorded in August and February, and the lower values were recorded in October and December (Tab. 2, Fig. 2).
Concentrations of TP ranged from 0.01 to 0.15 mg L−1, with a mean value of 0.04 mg L−1. Concentrations of SRP (soluble reactive phosphorus) were very low, almost undetectable. Concentrations of TN ranged between 0.05 and 3.94 mg L−1, and the main composition of DIN (dissolved inorganic nitrogen) was NO3-N. The TP and TN concentrations showed peaks in the wet season (August and March) and their lower concentrations were observed at the end of dry season (December) (Table 2, Fig. 3a, 3b).
Mean, interval (minimum and maximum) and standard deviation values of limnological variables (n = 12) at the five sampling stations in Zhushuqiao reservior.
 |
Fig. 2 Mean values of water temperature, SD and pH from April 2016 to February 2017 in Zhushuqiao reservoir. |
 |
Fig. 3 Mean concentrations of N and P from April 2016 to February 2017 in Zhushuqiao reservoir. |
3.2 Biotic variables
Average biomass and density were the highest in August, while decreased from October 2016 to February 2017. The quantity of phytoplankton in surface water was large, but it gradually reduced with the increase of water depth. Density and biomass of phytoplankton from surface to 10 m water layer were higher than other layers at the beginning of this study period.
Concentrations of biomass were similar at all sampling points, the minimum at 25 m of Site 5 in December 2016 and the maximum at 3 m of Site 2 in February 2017 (Fig. 4). Cryptophyta were the maximum biomass group before and after spring (February and April). This group was mainly dominated by Cryptomonas sp. Moving to June, the dominance shifted to Bacillariophyta and Pyrrophyta species by Synedra and Ceratium hirundinella. During August, Chlorophyta reached high biomass, accounting for 64% of the total biomass, with seasonal peaks of 5 mg L−1, and a mean value of 1.96 mg L−1. Pyrrophyta, Chlorophyta, Bacillariophyta, accounted for 85% of the total biomass in October. Bacillariophyta, Cryptophyta, and Chlorophyta accounted for 96% of the total biomass in winter (December). Concentrations of chlorophyll a were different at all periods, Chl-a concentrations varied largely (1.8–65.9 μg L−1), peaked from June to August, and in February, with mean concentrations of 36, 26 and 25 μg L−1, respectively. The lowest Chl-a value was observed in April. At the end of the dry season (October and December), the mean concentration of Chl-a was 12 and 7 μg L−1, respectively (Tab. 2, Fig. 4). Regarding biomass and chlorophyll a, no significant difference was found (p > 0.01) among the all sampling sites.
 |
Fig. 4 Mean values of biomass and Chl.a from April 2016 to February 2017 in Zhushuqiao reservoir. |
3.3 Temporal and spatial variations of CRS-survival strategists
Phytoplankton seasonal succession in the Zhushuqiao reservoir followed the sequence of X2+Y+F+Lo → Lo+D → F+P → F+Lo → C+Y+X2 → Y+X2+Lo, which showed characteristics of the cycle of dominant species. The phytoplankton functional group classification in this paper has been published in another paper by the authors (Huang, 2019). The spatial and temporal changes of phytoplankton survival strategies in the five sampling points were reflected in Figures 5 and 6.
In this paper the survival strategies change characteristics of dominant functional groups in the whole year as follows: C/CRS/CS strategists (April, Chlamydomonas sp., Cryptomonas sp., Oocystis sp.) → R/S strategists (June, Synedra sp., Peridinium sp.) → CS/R/S strategists (August, Oocystis, Staurastrum sp., Peridinium sp., Synedra sp.) → S/CS strategists (October, Peridinium sp., Oocystis sp.) → R/CRS strategists (December, Aulacoseira ambigua, Cryptomonas sp.) → S/CRS/C strategists (February, Peridinium, Cryptomonas sp., Chlamydomonas sp.). Regarding survival strategists, no significant difference was found (p > 0.01) among the five sampling sites.
The spatial and temporal changes of survival strategists in five sampling sites were no significant difference in each period. Overall vertical changes of survival strategies were greater in the upstream (S1 and S2) than the downstream (Fig. 6). The ordination of phytoplankton community based on survival strategies were presented in Figure 7. Similarity was smaller in April, December and February compared with other time periods. Overall, phytoplankton seasonal dynamic changes had high similarities in sites 3, 4 and 5, and the highest similarity among the 5 sites was in both August and December.
Sites 1, 3 and 5 had a high similarity in April 2016 (Fig. 7), the dominant growth strategies changed frequently at 10 m in sites 1 and 2, and the same pattern was observed for the 20 m in site 3. C-strategists and R-strategies were dominant at all depths, and CRS-strategists were dominant at all depths in site 1 and at the middle to bottom layers in sites 2 and 3 (Fig. 6a).The dominant species recorded in April showed morphological features of survival strategists, such as unicellular flagellates of functional group of Y (Cryptomonas sp., CRS-strategists), X2 (Chlamydomonas sp., C-strategists) and Lo (Peridinium sp., S-strategists), colonial non-flagellated of F (Oocystis sp., CS-strategists).
In June, a large vertical change of growth strategies was observed in site 1, while the water layer above 5 m changed frequently in other sites. A new dominant phytoplankton assembly in June that included a unicellular non-flagellated of functional group of D (Synedra sp., R-strategists), R-strategists were dominant at all depths (Fig. 6b). The colonial non-flagellated of F (Oocystis sp., CS-strategists) and additional component a large sized unicellular flagellated of Lo (Peridinium sp., S-strategists) were recorded. The maximum percentage of total biomass was Lo in sites 1 and 2, and F was also dominant in site 1. Sites 3 and 4 had a high similarity (Fig. 7), D was dominant in sites 3, 4 and 5, and it contributed to 80% of total biomass in site 5.
Sites 3, 4 and 5 had high similarities in both August and October 2016 with CS-strategies and R-strategies (Fig. 7). Chlorophyta was dominant in August, R/S/CS-strategists were dominant in all sites, while the colonial non-flagellated of F (Oocystis sp., CS-strategists) and D (Synedra sp., R-strategists), unicellular non-flagellated of P (Staurastrum sp., R-strategists), unicellular flagellated of Lo (Peridinium sp., S-strategists) were the dominant species. CRS-strategists were dominant at 25–30 m in site 3, at the middle water layer in site 4, and at the 5, 20 and 30m in site 5. C-strategists were also dominant in site 5 (Fig. 6c).
In October, a large sized unicellular flagellated of LO (Peridinium sp., S-strategists) were dominant in sites 1 to 4. A colonial non-flagellated of F (Oocystis sp., CS-strategists) and unicellular flagellates of Y (Cryptomonas sp., CRS-strategists) had high biomass in sites 3, 4 and 5 (Fig. 6d).
During December, sites 4 and 5 had a high similarity in December 2016 with CR-strategies (Fig. 7); non-flagellated of functional group of C (Aulacoseira ambigua, R-strategists) was dominant in all sites, followed by unicellular flagellates of Y (Cryptomonas sp., CRS-strategists). F (Oocystis sp., CS-strategists) was dominant in sites 3 and 4 (Fig. 6e).
Sites 3, 4 and 5 had a high similarity in February, with similar survival strategies as in April (Fig. 7). Unicellular flagellates of Y (Cryptomonas sp., CRS-strategists) was dominant in site 1, LO (Peridinium sp., S-strategists) was dominant in sites 2, 3 and 4. F (Oocystis sp., CS-strategists) was dominant in site 5. The dominant growth strategies changed frequently at 0.5m in sites 2, 3, 4 and 5 (Fig. 6f).
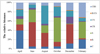 |
Fig. 5 The relative biomass of phytoplanton growth strategies in Zhushuqiao reservoir. |
 |
Fig. 6 The vertical variation of biomass and phytoplankton growth strategies. |
 |
Fig. 7 2-D NMDS ordinations of phytoplankton communities based on survival strategies among sampling sites in different water depths. |
3.4 RDA of phytoplankton growth strategies and environmental factors
There were 11 environmental factors (TP, TN, NO3-N, NO2-N, NH4-N, PO4-P, WT, DO, pH, SD, COD) and 7 phytoplankton growth strategies showed in the RDA. The ordination along axis 1 was statistically significant with an eigenvalue of 0.897. A total of 94.1% of the cumulative variance in species distribution was explained by the first two axes. The most effective explanatory factors were WT and DIN, both played a significant role in the seasonal succession of phytoplankton functional groups. Regarding phytoplankton growth strategies of CR, R and CS were located near to water temperature, PO4-P and SD in June and August. S-strategists were positively correlated with TP, TN, NO2-N, COD and pH in October. C-strategists were positively correlated with TN, DIN (NO2-N, NH4-N, NO3-N), TP, COD and pH in April, October and February. The phytoplankton growth strategies of CRS and R/CS were positively correlated with TN, DIN (NO2-N, NH4-N, NO3-N), TP, COD and pH in October. In addition, DO was far away from the phytoplankton growth strategies appeared at the top right of the diagram (Fig. 8).
 |
Fig. 8 Redundancy analysis of phytoplankton of C-R-S survival strategies related to environmental factors. |
4 Discussion
Due to the stratification effect of deep water reservoirs, the water environment will change in different seasons and at different depths, it is inadequate to study the variation of phytoplankton in the whole reservoir only in the surface water. This study through the analysis of the spatial and temporal changes of water environment and phytoplankton, it is concluded that the seasonal succession of dominant phytoplankton functional groups in Zhushuqiao reservoir was cyclic. The reason is that the seasonal dynamic characteristics of phytoplankton assemblages are often very typical and predictable from one year to the other in temperate regions (George et al., 2000; Salmaso and Padisák, 2007; Sommer et al., 2015). It is closely related to water depth, eutrophication and thermal stratification, and the most obvious one is the phytoplankton in the development of thermal stratification structure (Reynolds, 1999, 2006).
The vertical spatial dynamic variation of phytoplankton showed that the changes of dominant functional groups in each water layer were small at the same site. NMDS ordinations clearly showed that two adjacent sites had strong similarity, and the phytoplankton seasonal dynamic changes in sites 3, 4 and 5 had a high similarity in Zhushuqiao reservoir. The possible reason could be the similar hydrodynamics in two adjacent sites. The difference of phytoplankton in site 1 from others could be related to its geographical location and surrounding human disturbance. Hydrology was easily affected by human disturbance, and precipitation could be another important potential environmental driver (Melack, 1979; Lauri et al., 2012). Water depths in the upstream sampling sites were shallower than downstream, the water flowed faster, and its related human disturbance was larger than the downstream sites. These could explain the large vertical variations of phytoplankton in the upstream sites. C-strategist of X2 (Chlamydomonas), CRS-strategist of Y (Cryptomonas), and S-strategist of Lo (Peridinium) could adapt to light deficient condition and the swimming ability allowed them to explore various resources in restricted areas (Gasol et al., 1993; Reynolds and Irish, 1997; Giroldo and Vieira, 1999; Kruk et al., 2002; Reynolds, 2006). That could also explain that C-strategist and CRS-strategist were co-dominant in Site 1.
Seasonal succession of phytoplankton community is a complicated process that depends on a comprehensive effect of nutrient, disturbance patterns, water-retaining and regulating of reservoir, and sudden weather changes or other factors (Padisák et al., 2003). In the current study, we found key environmental factors were water temperature, SD, PO4-P, pH, TP, and DIN (P < 0.01). Water temperature is an important factor for seasonal changes of phytoplankton in previous studies (e.g., Padisák et al., 2003; Salmaso and Zignin, 2010). The development of diatoms in hot early summer (June) and low temperature winter (December) were observed in Zhushuqiao reservoir. The dominant species of Chlorophyta and Pyrrophyta of functional groups F, P and Lo were prefered in the high temperature of summer and autumn, while species of Chlorophyta, Cryptophyta and Pyrrophyta with groups X2, F, Y, and Lo during the mild of spring (February and April) were dominated.
The ratio of N/P is used for assessing nutrient limitations on phytoplankton growth (Becker et al., 2010). Our results showed that phosphorus was a limiting factor for total biomass of phytoplankton. The functional group of Y was dominant in the long period because it was significantly influenced by TP and DIN, as CRS-strategist was sensitive to phosphorus concentrations (Padisák et al., 2009). They are able to absorb phosphorus rapidly and gain fast growth (Albay and Akçaalan, 2003). Higher concentration of TN and TP during the summer periods along with R-strategist (functional groups of D and P) and S-strategist (Lo) indicated that the reservoir was in eutrophic conditions. In another study, Yang (2008) showed the growth of phytoplankton biomass responded to the increase concentration of N and P in Zhushuqiao reservoir. The C-strategist of group X2, dominated from winter to spring, had significantly positive relationships with DIN and TP but negative relationships with SD in this study. Usually, group X2 species are suited to exploit resource-saturated environments (Reynolds, 1987). X2 are sensitive to mixing and filter-feeding grazers (Reynolds et al., 2002; Padisák et al., 2009), and they were dominant in spring, and became less dominant during the summer and autumn. Increased grazing pressure from zooplankton (such as Daphnia) in summer and autumn could be one of the reasons for its reduction (Wang et al., 2011; Xiao et al., 2011). Group F were negatively correlated with TP (P < 0.005) and positively correlated with PO4-P and illumination intensity (P < 0.001) in this study, it has a wide adaptability to certain advantages of seasonal succession, especially, in August and October as thermal stratification proceeds. Epilimnion is often found having some dominant species, these species have the characteristics of rapid propagation, opportunist and neutrally buoyant, such as Oocystis of CS-strategist (Reynolds et al., 2002; Reynolds, 2006). This could also explain that group Lo, a nutrient stress tolerant of S-strategist species dominated the presence of K-selected (Reynolds, 1996). Large dinoflagellates, represented by the motile Peridinium, had greater biomass of species with high cell volumes in every stage, and usually found in stratification periods in mesotrophic lakes (Reynolds et al., 2002; Padisák et al., 2009). S-strategist are competitors, they can become dominant with abundant nutrients and energy, and the presence of flagella is helpful for them to obtain more nutrients from the deep layer (Borics et al., 2005; Lopes et al., 2005). Lo are often dominant in summer and autumn (Padisák et al., 2003; Wang et al., 2011), and these two periods share some similar environmental conditions, such as water temperature, stratification of water column, and nutrients (Huszar et al., 2003). The storm and shorter water retention time does not ensure cell division of Peridinium circyum in unstable condition (Pollingher and Zemel, 1981; Xiao et al., 2011).
In this study, the dominance of R-strategist (D and P) was found in June, which was consistent with some previous studies (Moreno-Ostos et al., 2008; Padisák et al., 2003). The representative of D is Synedra, which has flushing tolerances. Group D was negatively correlated with TN (P < 0.001) and DIN (P < 0.005), and positively correlated with TP and water temperature (P < 0.001) in this study period. Increased temperatures, light intensity and nutrient concentration of phosphorus, and beginning of stratification in early summer made group D predominant. Group P had greater biomass of species Staurastrum with high cell volumes, its habitat template at higher trophic states water body (Padisák et al., 2009) and in the epilimnion of stratification lakes when the water mixing was satisfied (Padisák et al., 2009). Increased temperature (P < 0.005) and PO4-P (P < 0.001) were major driving factor reason for group P predominant. Several potential points could account for this. Firstly, group D and P fell into the same category of R-strategists, they were elongate and large dimensions in shape, and they had the ability to capture light energy under high mixing and nutrient conditions (Alvesdesouza et al., 2008). Secondly, R-strategist species had the ability of drifting in the mixing water layer, tolerating disturbance and reconciling or accommodating strategists (Smayda and Reynolds, 2003). Thirdly, most species of phytoplankton preferred summer when more light penetration and higher temperatures were available (Wilk-Woźniak and Żurek, 2006). The lower temperatures reduced phytoplankton swimming speed (Heaney and Eppley, 1981).
CR-strategist of group C (Aulacoseira ambigua) was favorable in winter. Group C was dominant only during the winter in this reservoir when the temperature and nutrition concentration were the lowest, which prefers to R-strategist in Zhushuqiao reservoir. In this study, group C was significantly negative related with water temperature, nitrogen and phosphorus. This could explain that the temperature is an important ecological factor as low temperatures are favorable for some diatoms species during winter (Moreno-Ostos et al., 2008). Diatoms have a wide range of temperature tolerance (Wilk-Woźniak and Żurek, 2006), and their advantage was showed when water temperature below 18 °C (Silva et al., 2005). Group C was favorable in low water temperature, low light and mixing conditions (Lopes et al., 2005, Tolotti et al., 2007; Stević et al., 2013), which could be beneficial for them to compete with other functional groups in winter (Wang et al., 2011). Group C were adapted to low light availability and sensitive to stratification (Padisák et al., 2003).
Overall, different survival strategists of dominant species were presented in every season. On one hand, it was not one single strategist; instead, there were two or more strategists to adapt to environment changes, in order to live in different periods. On the other hand, the different dominant species or community had coexistence mechanisms of performing complementary strategies during the same period. Therefore, in spring, stress-tolerant and disturbance-tolerant species of CRS-strategist were dominant, they adapted to low temperature, light and nutrient. Small individual of opportunistic colonists of C-strategist were dominant before the stratification. With the increase of nutrient and the rise of temperature, slightly bigger, disturbance-tolerant species of R-strategist and those adapted to stratification and stress-tolerant species of CS-strategy were dominant. During the winter, some species (i.e., the R-strategists) adapted to low temperature were dominant in the Zhushuqiao reservoir.
5 Conclusions
In this study, phytoplankton functional groups seasonal succession followed the sequence of X2+Y+F+Lo → Lo+D→ F+P → F+Lo→ C+Y+X2→ Y+X2+Lo. Corresponding growth strategies were as follows: C+CRS+CS+S→ S+R→ CS+R→ CS+S→ CR+CRS+ C→ CRS+C+S. Seasonal succession showed cyclical changes in phytoplankton assemblages, and two adjacent sites had strong similarities.
Water temperature, pH, TP and DIN were key factors in the selection of phytoplankton functional groups of growth strategies succession, water temperature were likely to be the critical factors affecting phytoplankton communities in later spring to summer.
In the Zhushuqiao reservoir, we may need to pay more attention to high water temperature, the number of phytoplankton that increased dramatically, such as Synedra, Cryptophyta, and Peridinium, which could have the potential to form blooms.
Acknowledgements
This work was supported by the [Chinese Academy of Sciences #1] under Grants [numbers Y623021201, Y45Z041201, and ZDRW-ZS-2017-3-2]; and the scientific research funds of Guiyang University [GYU-KY-(2020)].
References
- Alvesdesouza C, González MT, Iriarte JL. 2008. Functional groups in marine phytoplankton assemblages dominated by diatoms in fjords of southern Chile. J Plankton Res 30: 1233–1243. [Google Scholar]
- Albay M, Akçaalan R. 2003. Factors influencing the phytoplankton steady state assemblages in a drinking-water reservoir (Őmerli reservoir, Istanbul). Hydrobiologia 502: 85–95. [CrossRef] [Google Scholar]
- Alvesdesouza C, Menezes M, Huszar V. 2006. Phytoplankton composition and morphological functional groups in a tropical humic coastal lagoon, Brazil. Acta Bot Bras 20: 78–83. [Google Scholar]
- Arauzo M, Cobelas MA. 1994. Phytoplankton strategied and time scales in a eutrophic reservoir. Hydrobiologia 291: 1–9. [Google Scholar]
- Arrigo KR. 2005. Marine microorganisms and global nutrient cycles. Nature 437: 349–355. [CrossRef] [PubMed] [Google Scholar]
- Becker V, Caputo L, Ordóñez J, et al. 2010. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res 44: 3345–3354. [PubMed] [Google Scholar]
- Borics G, Grigorszky I, Padisák J, Barbosa FAR, Doma ZZ. 2005. Dinoflagellates from tropical Brazilian lakes with description of Peridinium brasiliense sp. nova. Algol Stud 118: 47–61. [Google Scholar]
- Bray JR, Curtis CT. 1957. An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27: 325–349. [CrossRef] [Google Scholar]
- Carney HJ, Goldman CR. 1988. Seasonal phytoplankton r- and K-selection in oligotrophic Lake Tahoe. Verh Internat Verein Limnol 23: 672–676. [Google Scholar]
- Crossetti LO, Bicudo CEM. 2008. Adaptaions in phytoplankton life strategies to imposed change in a shallow urban tropical eutrophic reservoir, Garças Reservoir, over 8 years. Hydrobiology 614: 91–105. [Google Scholar]
- Gasol JM, Garcíacantizano J, Massana R, Guerrero R, Pedrósalió C. 1993. physiological ecology of a metalimnetic Cryptomonas population: relationships to light, sulfide and nutrients. J Plankton Res 15: 255–275. [Google Scholar]
- George DG, Talling JF, Rigg E. 2000. Factors influencing the temporal coherence of five lakes in the English Lake District. Freshw Biol 43: 449–461. [Google Scholar]
- Giroldo D, Vieira AAH. 1999. Assimilation of 14C in a tropical strain of Criptomonas obovata (Cryptophyceae) exposed to several irradiances. J Plankton Res 21: 1911–1921. [Google Scholar]
- Grime P. 1979. Plant strategies and vegetation processes. Chichester, England: Wiley-Intescience Press, pp.1–222. [Google Scholar]
- Hambright KD, Zohary T. 2000. Phytoplankton species diversity control through competitive exclusion and physical disturbances. Limnol Oceanogr 45: 110–122. [Google Scholar]
- Heaney SI, Eppley RW. 1981. Light, temperature and nitrogen as interacting factors affecting diel vertical migrations of dinoflagellates in culture. J Plankton Res. [Google Scholar]
- Hillebrand H, Dürselen CD, Kirschtel U, Pollingher D, Zohary T. 1999. Biovolume calculation for pelagic and benthic microalgae. J Phycol 35: 403–424. [CrossRef] [Google Scholar]
- Hu HJ, Wei YX. 2006. The Freshwater Algae of China Systematics, Taxonomy and Ecology. Beijing (CN): Science Press (in Chinese). [Google Scholar]
- Hu R, Li Q, Han BP, Naselli-Flores L, Padisak J, Salmaso N. 2015. Tracking management-related water quality alterations by phytoplankton assemblages in a tropical reservoir. Hydrobiology 763: 109–124. [Google Scholar]
- Huszar V, Kruk C, Caraco N. 2003. Steady-state assemblages of phytoplankton in four temperate lakes (NE U.S.A.). Hydrobiology 502: 97–109. [Google Scholar]
- Kruk C, Mazzeo N, Lacerot G, Reynolds CS. 2002. Classification schemes for phytoplankton: a local validation of a functional approach to the analysis of species temporal replacement. J Plankton Res 24: 901–912. [Google Scholar]
- Kruk C, Segura AM. 2012. The habitat template of phytoplankton morphology-based functional groups. Hydrobiology 698: 191–202. [Google Scholar]
- Kruskal JB. 1964. Nonmetric multidimensional scaling: a numerical method. Psychometrika 29: 115–129. [CrossRef] [MathSciNet] [Google Scholar]
- Lauri H, Moel HD, Ward PJ, Räsänen TA, Keskinen M, Kummu M. 2012. Future changes in mekong river hydrology: impact of climate change and reservoir operation on discharge. Hydrol Earth Syst Sci 16: 4603–4619. [Google Scholar]
- Lopes MRM, Bicudo CEM, Ferragut MC. 2005. Short term spatial and temporal variation of phytoplankton in a shallow tropical oligotrophic reservoir, southeast Brazil. Hydrobiology 542: 235–247. [Google Scholar]
- Margalef R. 1978. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanologia 1: 493–509. [Google Scholar]
- Melack JM. 1979. Temporal variability of phytoplankton in tropical lakes. Oecologia 44: 1–7. [PubMed] [Google Scholar]
- Moreno-Ostos E, Cruz-Pizarro L, Basanta A, George DG. 2008. The spatial distribution of different phytoplankton functional groups in a Mediterranean reservoir. Aquat Ecol 42: 115–128. [CrossRef] [Google Scholar]
- Naselli-Flores L, Padisák J, Albay M. 2007. Shape and size in phytoplankton ecology: do they matter? Hydrobiology 578: 157–161. [Google Scholar]
- Padisák J, Borics G, Fehér G, et al. 2003. Dominant species, functional assemblages and frequency of equilibrium phases in late summer phytoplankton assemblages in Hungarian small shallow lakes. Hydrobiology 502: 157–168. [Google Scholar]
- Padisák J, Crossetti LO, Naselli-Flores L. 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiology 621: 1–19. [Google Scholar]
- Pollingher U, Zemel E. 1981. In situ and experimental evidence of the influence of turbulence on cell division processes of Peridinium cinctum forma westii (Lemm.) Lefèvre. Eur J Phycol 16: 28–287. [Google Scholar]
- Reynolds CS, Irish AE. 1997. Modelling phytoplankton dynamics in lakes and reservoirs: the problem of in-situ growth rates. Hydrobiology 349: 5–17. [Google Scholar]
- Reynolds CS. 1987. The response of phytoplankton communities to changing lake environments. Swiss J Hydrol 49: 220–236. [Google Scholar]
- Reynolds CS. 1988. Functional morphology and the adaptive strategies of freshwater phytoplankton. In Sandgren, Craig D, editors. Growth and Reproductive strategies of freshwater phytoplankton. Cambridge (UK): Cambridge University Press, pp. 388– 433. [Google Scholar]
- Reynolds CS. 1996. The plant life of the pelagic. Verh Internat Verein Limnol 26: 97–113. [Google Scholar]
- Reynolds CS. 1997. Vegetation in the pelagic: a model for ecosystem theory. In: Kinne O, editors. Excellence in Ecology, Vol. 9. Germany: Ecology Institute. p. 371 [Google Scholar]
- Reynolds CS. 1999. Phytoplankton assemblages in reservoirs. In: Tundisi JG, Straskraba M, editors. Theoretical reservoir ecology and its applications. São Carlos (BRA): International institute of ecology, Brazilian academy of sciences and Backhuys Publishers. p. 439– 456. [Google Scholar]
- Reynolds CS. 2006. Ecology of phytoplankton. Cambridge, England: Cambridge University Press (UK), pp. 1–535. [Google Scholar]
- Reynolds CS, Huszar V, Kruk C, Naselliflores L, Melo S. 2002. Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24: 417–428. [Google Scholar]
- Salmaso N, Naselli-Flores L, Padisák J. 2014. Functional classifications and their application in phytoplankton ecology. Freshw Biol 60: 603–619. [Google Scholar]
- Salmaso N, Padisák J. 2007. Morpho-Functional Groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiology 578: 97–112. [Google Scholar]
- Salmaso N, Zignin A. 2010. At the extreme of physical gradients: phytoplankton in highly flushed, large rivers. Hydrobiology 639: 21–36. [Google Scholar]
- Salmaso N. 1996. Seasonal variation in the composition and rate of change of the phytoplankton community in a deep subalpine lake (Lake Garda, Northern Italy). An application of nonmetric multidimensional scaling and cluster analysis. Hydrobiology 337: 49–68. [Google Scholar]
- Sandgren SD. 1988. The ecology of chrysophyte flagellates: their growth and perenation strategies, as freshwater phytoplankton. In Sandren CD eds, Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge (UK): Cambridge University Press, pp. 9– 104. [Google Scholar]
- Santos ACAD, Calijuri MC. 1998. Survival strategies of some species of the phytoplankton community in the Barra Bonita Reservoir (São Paulo, Brazil). Hydrobiology 367: 139–151. [Google Scholar]
- Scheffer M, Rinaldi S, Gragnani A, Mur L, Nes EHV. 1997. On the dominance of filamentous Cyanobacteria in shallow, turbid lakes. Ecology 78: 272–282. [CrossRef] [Google Scholar]
- Scheffer M, Rinaldi S, Huisman J, Weissing FJ. 2003. Why plankton communities have no equilibrium: solutions to the paradox. Hydrobiology 491: 9–18. [Google Scholar]
- Silva CAD, Train S, Rodrigues LC. 2005. Phytoplankton assemblages in a Brazilian subtropical cascading reservoir system. Hydrobiology 537: 99–109. [Google Scholar]
- Smayda TJ, Reynolds CS. 2001. Community assembly in marine phytoplankton: application of recent models to harmful dinoflagellate blooms. J Plankton Res 23: 447–461. [Google Scholar]
- Smayda TJ, Reynolds CS. 2003. Strategies of marine dinoflagellate survival and some rules of assembly. J Sea Res 49: 95–106. [Google Scholar]
- Sommer U, Adrian R, Domis LDS, et al. 2015. Beyond the plankton ecology group (PEG) model: mechanisms driving plankton succession. Annu Rev Ecol Evol Soc 43: 429–448. [Google Scholar]
- Sommer U. 1981. The role of r- and K-selection in the succession of phytoplankton in Lake Constance. Acta Oecol Oecol Gen 2: 327–342. [Google Scholar]
- Stević F, Mihaljević M, Špoljarić D. 2013. Changes of phytoplankton functional groups in a floodplain lake associated with hydrological perturbations. Hydrobiology 709: 143–158. [Google Scholar]
- Sun J, Liu DY, Qian SB. 1999. Study on phytoplankton biomass I, phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanol Sin 21: 75–85. [Google Scholar]
- Tolotti M, Corradini F, Boscaini A, Calliari D. 2007. Weather-driven ecology of planktonic diatoms in Lake Tovel (Trentino, Italy). Hydrobiology 578: 147–156. [Google Scholar]
- Wang L, Cai Q, Xu YY, Kong L, Tan L, Zhang M. 2011. Weekly dynamics of phytoplankton functional groups under high water level fluctuations in a subtropical reservoir-bay. Aquat Ecol 45: 197–212. [CrossRef] [Google Scholar]
- Wilk-Wozniak E. Żurek R. 2006. Phytoplankton and its relationships with chemical parameters and zooplankton in meromictic Piaseczno reservoir, Southern Poland. Aquat Ecol 40: 165–176. [Google Scholar]
- Xiao LJ, Wang T, Hu R, Han BP, Wang S. 2011. Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large canyon-shaped reservoir. Water Res 45: 5099–5109. [CrossRef] [PubMed] [Google Scholar]
- Yang XL. 2008. Study on the community characteristics of zooplankton in Zhushuqiao reservoir and biological evaluation of water quality [master's thesis]. Hunan (CN): Central south university of forestry and technology. [Google Scholar]
Cite this article as: Huang G, Wang X, Chen Y, Deng L, Xu D. 2021. Survival strategies of phytoplankton functional groups to environmental factors in a drinking water reservoir, central China. Ann. Limnol. - Int. J. Lim. 57: 18
All Tables
Mean, interval (minimum and maximum) and standard deviation values of limnological variables (n = 12) at the five sampling stations in Zhushuqiao reservior.
All Figures
 |
Fig. 1 Location of Zhushuqiao Reservoir and the sampling sites. |
| In the text | |
 |
Fig. 2 Mean values of water temperature, SD and pH from April 2016 to February 2017 in Zhushuqiao reservoir. |
| In the text | |
 |
Fig. 3 Mean concentrations of N and P from April 2016 to February 2017 in Zhushuqiao reservoir. |
| In the text | |
 |
Fig. 4 Mean values of biomass and Chl.a from April 2016 to February 2017 in Zhushuqiao reservoir. |
| In the text | |
 |
Fig. 5 The relative biomass of phytoplanton growth strategies in Zhushuqiao reservoir. |
| In the text | |
 |
Fig. 6 The vertical variation of biomass and phytoplankton growth strategies. |
| In the text | |
 |
Fig. 7 2-D NMDS ordinations of phytoplankton communities based on survival strategies among sampling sites in different water depths. |
| In the text | |
 |
Fig. 8 Redundancy analysis of phytoplankton of C-R-S survival strategies related to environmental factors. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


