| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 56, 2020
|
|
|---|---|---|
| Article Number | 14 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/limn/2020012 | |
| Published online | 04 June 2020 | |
Research Article
Status of fish biodiversity and fishing on Hau River, Mekong Delta, Vietnam
College of Aquaculture and Fisheries, Can Tho University, Can Tho, Vietnam
* Corresponding author: vnut@ctu.edu.vn
Received:
15
March
2020
Accepted:
12
May
2020
Fish biodiversity on Hau (Bassac) river was investigated to assess the status of species composition and fishing by fishing gears during a year. Sampling was implemented monthly at the upper part (An Giang province), middle part (Can Tho City) and lower part (Soc Trang province) of Hau River using trawl net as main sampling gear. Additionally, fish composition was also recorded from four other most popular fishing gears including cast net, gill net, fixed net and hook operated in the study sites. Fish species composition was determined by fishing gears and their abundance (CPUE) was calculated only from the main sampling gear (trawl net). The results showed that a total of 176 fish species belonging to 16 orders and 49 families was recorded. Perciformes was the most abundant group with 51 species followed by Cypriniformes with 46 species. The number of fish species was decreasing from upper part to lower part. Trawl net was considered the most destructive gear as up to 145 fish species caught by this device, followed by gill net with 98 species, fixed net 75, cast net 57, and hooks 16 species. CPUE was very low ranging from 0.53 kg.ha−1 h−1 to 26.30 kg.ha−1 h−1. Higher CPUE was recorded at lower part in compared to upper part and middle part, and at dry season in compared to rainy season. Regulation on fishing gears, fishing ground and season should be taken into consideration to protect and conserve the resources.
Key words: Fish diversity / catch per unit effort (CPUE) / fishing gears / Hau River / trawl net
© EDP Sciences, 2020
1 Introduction
The Mekong River has the second highest biodiversity of any river after Amazon with more than 1000 fish species recorded (Coates et al., 2005; Baran, 2010). Over more than 4000 km, Mekong River runs through 6 countries starting from Tibet and ending to the East Sea in the Mekong Delta where it splits into the Bassac and Mekong rivers (as Hau and Tien river in Vietnamese, respectively). A number of studies have investigated fish biodiversity and fishing status on the Mekong River basin (Valbo-Jørgensen et al., 2009; Nakano et al., 2012) and Baran (2010) also showed the changes in fish biodiversity and fisheries status along the length of the whole Mekong River. Such diversity has made the Mekong River an extremely important source of food for more than 70 million people living along the river. However, overexploitation, pollution, habitat alternation, upstream dam construction and other factors have caused serious reduction in fish biodiversity and productivity, especially in the lower basin of the river (Coates et al., 2005). Many economically important and endemic fish species have become endangered species and been increasingly proposed for protection and conservation. Poulsen et al. (2004) mentioned 40 important fish species in the Mekong lower basin of which 7 species are endemic and 3 further species are endangered to the point of extinction. Tenualasa thibaudeaui, Cirrhinus lobatus, Hampala dispar, Pangasianodon gigas, Puntioplitas falcifer, Probarbus jullieni and Aaptosyax gypus are important endemic species for requiring protection. The authors also warned of the likely disappearance of 3 species including Laotian shad (Tenualasa thibaudeaui), Isok barb (Probarbus jullieni) and Giant catfish (Pangasianodon gigas). Coates et al. (2005) showed the most destructive fishing gear to be the bag net or Dai that can capture 40 species in a single catch.
Hau River, also known as Bassac River, is the largest branch of the Mekong River with a length of 226 km, the river flows through 4 provinces in the Mekong Delta (MD) including An Giang (river length 104 km), Can Tho (48km), Hau Giang (15 km) and Soc Trang (59 km). However, the substantial aquatic resources, which contribute considerably to the freshwater fishery production of the region, has been shown to be declining (Berra, 2001; Pitcher and Hollingworth, 2002; Nguyen et al., 2007). A study conducted in the upper part of the river (An Giang province) revealed that 7 species had an endangered status and could not be regularly encountered including Pangasianodon gigas, Pangasius sanitwongsei, Dasyatis laosensis, Pristis microdon, Chitala blanci, Tenualosa thibaudeaui, and Orcaella brevirostris (Nguyen et al., 2007). Although there are studies on fish composition in Hau River, there is no information on the status of the fisheries. Little is known about the types of fishing gear, or their impacts for example catch composition or catch per unit effort by gear in the Hau River.
The aim of this study was to investigate diversity and fishing status of fish on the Hau River to provide an important baseline for proper monitoring and managing the resources in the Bassac River, lower Mekong river basin to further biodiversity conservation steps in this important part of the Mekong River basin.
2 Methods
2.1 Sampling sites
Along Hau River, 3 main zones were selected for sampling including An Giang (upper part)-the border to Cambodia, Can Tho (middle part) and Soc Trang (lower part) − the river mouth. At each zone, 2 sampling sites were chosen as depicted in Table 1 and Figure 1.
Sampling sites on Hau river.
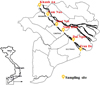 |
Fig. 1 The selected sampling sites on Hau River. |
2.2 Sampling methods
Fish samples were directly collected by trawl net which was operated regularly on the monthly basis at the 6 sampling sites in the full moon period. The trawl net with a length of 10 m and smallest mesh size at the end part of 1 cm was designed with an iron frame of 0.5 m high and 3.5 m wide and dragged over the bottom of the river for approximately 20 minutes at a rate of 4–5 km h−1. In addition to direct sampling, fish were also collected from other fishing gears including trammel nets, fixed nets, lift nets and line-hooks which were being operated at the same time in the surrounding area. The sample collection from other gears was conducted monthly on the same day as the direct sampling for each site and determined fish composition or diversity solely. Fish were preserved on ice during transfer to the laboratory of College of Aquaculture and Fisheries, Can Tho University for analysis.
All fish specimens were morphologically measured with total length and standard length and number of dorsal fin, pelvic fin, pectoral fin, lateral scale (Fig. 2) was counted. Based on these parameters, identification of fish species was made using common taxonomic keys such as Vuong (1954–1955), Nguyen (1991), Truong and Tran (1993), Rainboth (1996) and Tran et al. (2013). The conservation status of each fish species was assessed using the IUCN Red list (IUCN, 2019).
Total number of fish species was determined and comparison of fish composition was made for each zone. Number of fish species was recorded for each type of fishing gear to determine the most destructive one. Fish abundance at different zones was estimated using trawling catch per unit effort (CPUE, kg ha−1 h−1).
The Venn diagram, which was used to find out the shared species among sampling areas and among fishing gears, was conducted by Draw Veen Diagram online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
All statistical analyses were performed in the R-environment (https:// www.r-project.org) unless otherwise indicated.
 |
Fig. 2 Morphological parameters used to measure for species identification. |
3 Results
3.1 Fish diversity on Hau River
A total of 176 species belonging to 16 orders, 97 genera and 49 families were recorded at three different sampling zones across the Hau River. A list of species recorded at each zone of Hau River is provided in Table A1. Highest number of species was found in the orders of Siluriformes, Cypriniformes, and Perciformes, accounting for 19.77, 25.99 and 28.81% of the total number of species found, respectively (Fig. 3A). The species number decreased from the upper part to the lower more saline estuarine zone. In the upper part, a total of 134 species belonging to 13 orders and 38 families were found; the middle part was characterized by 111 species belonging to 12 orders and 36 families. Lower number of fish species was recorded in the lower part with 104 species belonging to 11 orders and 40 families. The species number in the three most common orders changed according to zones. The proportion of the total number of species was greatest in the Cypriniformes in the upper (31%) and middle (32%) zones of the river but Perciformes dominated in the lower zone (40.38%) (Fig. 3B–D).
The Shannon-Weiner diversity index of fish in the upper part, middle part and lower part of Hau River estimated using only trawl net catches demonstrated a strong relationship with overall species richness. Mid part had a lower fish diversity index (4.08) as compared to upper (4.33) and lower part (4.10) as shown in Table 2. A high species evenness index (0.93) was found across all the sampling zones of Hau River.
Among the recorded species, 25 species are commercially exploited and economically valuable. One endangered species Isok barb Probarbus jullieni Sauvage, 1880 (Cypriniformes) was recorded in both upper part and middle zones of the river; but six vulnerable species including small scale mud carp Cirrhinus microlepis Sauvage, 1878, Cosmochilus harmandi Sauvage, 1878, siamese algae-eater Gyrinocheilus aymonieri Tirant, 1883 (Cypriniformes), four-barred tiger fish Datnioides quadrifasciatus Sevastianov, 1809, spotted archerfish Toxotes chatareus Hamilton, 1822 (Perciformes), and laotian shad Tenualosa thibaudeaui Durand, 1940 (Clupeiformes) were rarely recorded. Alien species including sucking fish or vermiculated sailfin catfish (Pterygoplichthys disjunctivus Weber, 1991) and pirapitinga (Piaractus brachypomus Cuvier, 1818) were found in most areas of the river.
The Venn diagram shows that 58 species were found in all 3 sampling zones, representing 32.95% of the total species found in the Hau River (Fig. 4A). Of the species unique to a zone, 21 species (11.93%) were found only in the upper zone, 6 species (3.40%) in the middle zone and 34 species (19.32%) only in the lower zone. The most vulnerable species to capture were those five species caught by all five fishing gears including Puntius orphoides Valenciennes 1842 (Cypriniformes), Ompok bimaculatus Block 1794, Micronema bleekeri Bocourt 1866, Pangasius larnaudii Boucourt 1866, Pangasius macronema Bleeker 1850 (Siluriformes) (Fig. 4B). Trawling caught the highest number of species (145 species) and highest number of unique species captured (55 species). Gill net caught the second highest number of species (98 species) of which 17 were unique species caught, followed by cast net, fixed net, and line-hook. The endangered species Isok barb or Jullien's golden carp Probarbus jullieni was caught by only gill net. The vulnerable species, spotted archerfish Toxotes chatareus was captured by trawl net, gill net and cast net; Cosmochilus harmandi and four-barred tiger fish Datnioides polota were caught by only trawl net; small scaled mud carp Cirrhinus microlepis was caught by trawl net and gill net, and siamese algae-eater Gyrinocheilus aymonieri and laotian shad Tenualosa thibaudeaui were captured by only gill net.
 |
Fig. 3 The proportion of the total number of species by order of fish in the Hau River. A Overall, B Upper part, C Middle part, and D Lower part. |
Indices of fish community structure by trawl net in HAU River.
 |
Fig. 4 The Venn diagrams showing the unique and shared species between different zones (A) and fishing gears (B). |
3.2 Seasonal patterns in fish abundance (CPUE)
Highest abundance of fish was evidenced by highest CPUEs in periods of December and January in the lowest estuarine zones where 26.3 and 22.4 kg ha−1 h−1 obtained respectively (Fig. 5). Greatest fish abundance was also recorded in January in the upper and middle zones, with 20.3 and 10.5 kg ha−1 h−1 caught, respectively. Fish abundance was consistently greater in the lower zone with mean abundance of 5.04 kg ha−1 h−1, compared to the middle (0.53 kg ha−1 h−1) and upper (1.59 kg ha−1 h−1) zones.
Mean abundance was higher in the dry season (November to April) than in the rainy season (May to October) at all zones. Lowest mean CPUE (3.26 kg ha−1 h−1) was found at the upper part in the rainy season, and highest mean CPUE (15.52 kg ha−1 h−1) was at recorded in the lower zone in the dry season.
 |
Fig. 5 CPUE estimated throughout the year by trawl net in 3 sampling zones on Hau River. |
4 Discussions
The Mekong River basin is known for its richness in fish diversity but the total number is complicated by rare species and because of the un-surveyed areas and the marine species in the lower river (Valbo-Jørgensen et al., 2009). Vidthayanon (2008) listed 461 fish species in the pictorial book of the Mekong Delta. Wider research conducted by Valbo-Jørgensen et al. (2009) recorded 924 species (898 indigenous) on the Mekong Fish Database. Those species belong to 24 orders and 87 families that may exceed most other rivers in the world. Within the Mekong Delta, Tran et al. (2013) reported 322 species belonging to 77 families in a survey that covered all types of water bodies or ecosystems in the Mekong Delta,. The scope of the present study was much narrower focusing on only five main fishing gears deployed in the mainstream of the Hau River and explains the lower number of species (176 species) found. However, the composition of fish in this study was consistent with other studies in that the most abundant orders found in the rivers are Cypriniformes, Perciformes, and Siluriformes.
Fish species compositions in three zones in this study were broadly similar, with the greatest similarity found between the middle part and upper part or lower part. The greatest number of unique species were found in the lower and upper parts, was 34 and 21 species, respectively and is probably explained by salinity effects between the complete freshwater upper part and more brackish water lower part. Vidthayanon (2008) identified over 160 freshwater species which were the major populations in the upper zone and 89 freshwater fish species that can tolerate brackish water and live within the estuary in the Mekong Delta. Some 80 marine fish species may also occur in the estuary of the Mekong Delta (Vidthayanon, 2008), although Valbo-Jørgensen et al. (2009) reported only 47 marine species in the Mekong Fish Database.
Fisheries vary greatly from region to region, depending on the availability and access to markets. In areas with more abundant resources and greater human populations such as the delta area of Viet Nam, fishery is likely to be the most heavily exploited (Hortle, 2009). Local people in the area have been transferred experiences from generation to generation as to which of the many different fishing methods can be used to catch fish from the rivers, small channels, wet-land areas or even on the rice fields (Deap et al., 2003). Most of fishing gears and fishing activities are of traditional design and manufactured with extensive use of local material (Welcomme, 2001). There are hundreds of fishing gears belonging to 16 main groups typically used in the Mekong River (Claridge et al., 1997; Deap et al., 2003; Nguyen et al., 2006). The most popular methods, therefore, are operated at small-scale and household level with the involvement of all ages and genders. The low cost of most fishing gears, plus the part-time nature of most fishing, promotes great flexibility in the fishery. The number of people engaging inland fisheries can expand and contract very rapidly in response to natural variations in fish abundance by seasonal. Secretariat (1992) pointed out the continuing weak information base, the unreliability of official statistics based on estimates of commercial catches, and lack of data on artisanal fisheries, which might be contributing an unmeasured 80–95% of total catches. The seasonal nature and underreporting of catches presents many difficulties for research and could have had considerable impact on past studies. The scope of this study was focused on the mainstream of the Hau River with the main fishing gear − trawl net and the four gears with only species diversity information purposes. Those fishing gears can be considered as common commercial fisheries that do occur in rivers, and particularly in the lower Mekong basin. Trawl net is the only gear that can be moved to catch fish along the river while the others are more sedentary. This advanced characteristic in a combination with smaller average mesh size explain its effectiveness in fishing. As a consequence, up to 145 fish species regardless of others were caught as compared to the other gears which caught much less species. However, ecologically this is considered a devastating and destructive gear to the resources, especially when electricity is embedded. Trawl nets are listed as destructive and illegal fishing gears in Cambodia and Laos and legally prohibited (MRC, 2017). Trawl nets are used commonly in the mainstream and large tributaries of the Hau River. Most of them are unfortunately associated with electricity (per. communication) that can damage seriously the resources. After trawl nets, gill nets, especially trammel nets are of destructive gear too. In a study on assessment of gill nets and other fishing gears used in the Mekong river between Kratie and the Laos border, Cheng and Nam (2011) revealed that gill nets are commonly used in the region and their targets are the migrating fish and even dolphin. This also explains why gill nets are the second fishing gear catching more fish species right after trawl nets. Regulations on proper use of these fishing gears together with seasonal should be strongly concerned and monitored by the local authority.
Similar results from the studies of Baran et al. (2005) and Ngor et al. (2006) demonstrated that typically around 10 species would make up to about 60–70% of the total catch by weight, although the number of species caught could be up to 200 species. The CPUE in the region has been declining even as the total catches continue to increase with the intensifying fishing pressure (Welcomme, 2001). Declining CPUE has happened not only in the Hau River area but also throughout the Lower Mekong Basin (Phan et al., 2002; Hortle and Suntornratana, 2008). While there is no evidence of any decline in overall fish catch as yet, there has been a decline in large fish species (Mattson et al., 2002). Those large fish are more susceptible to fishing pressure because of their long lives and the length of time they take to reach reproductive size. Also, many of the methods used to catch smaller species will catch large species such as trawl net. The CPUE in figures 4 and 5 indicated rather similar trend of CPUE at three sampling zones in the year. This could be due to the geographical characteristic of the delta and the short distance between zones. However, the CPUEs in the lower zone were higher than that of other two zones in most of sampling sites. According to Valbo-Jørgensen et al. (2009), many fish species in lowlands of Mekong river including some estuarine fishes are forced downstream when the flow is low, and many fish species in Mekong river have the habit of migrating downstream. Additionally, according to Hortle (2009) many coastal and estuarine fish species can move considerable distance to the river system. This indicated the lower part or downstream zone of Hau River has also high diversity of fish species as well as high contribution to the inland fisheries in the region. Furthermore, the large seasonal and the variations of water availability in the Hau River normally change the habitats of fish. Many fishes often move to floodplain areas where are filled by rainwater or flood leading to less number of fish in the mainstream; in contrast, those fishes have to return to mainstream or to be trapped in floodplain area in the dry season (Hortle, 2009). This downstream migration possibly explains why the CPUEs in the dry season were higher than that in the rainy season along the Hau River (Figure 6).
The recorded composition and distribution of fish species in this study is suitable for the migrating and spawning activities of fishes in this river. Hortle (2009) mentioned about the migration of the white fish which are categorized as fish associating mainly with flowing water in the Mekong River − the fish species have habit of migrating long distance from floodplain into and along main river channels. Those fish species usually migrates into floodplain areas and downstream for spawning during the rainy or flooding season. After one month of the dry season, the juvenile fish have grown sufficiently to return to the main river from the floodplain areas as the flood waters retreat and floodplain areas dry-off. At this time these migratory fishes are ready to return to the upstream zone. This activity may help explain by the positive correlations (higher number of species catch) between middle zone and upper zone with the sampling sites in December − beginning of the dry season.
 |
Fig. 6 Boxplot for the CPUE by trawl net in two seasons (dry and rainy) along Hau River. |
5 Conclusions
Total fish species recorded in the Hau River by five main fishing gears was 176 species belonging to 49 families and 16 orders. Trawl net is the most effective gear followed by gill net in catching a wide variety of species and are commonly used year round. Most of the endangered and vulnerable fish species on Hau River were caught by these two gears. They are therefore considered the most destructive fishing gears and need to be regulated to a certain extent by local authority to protect and conserve the fish resources. The occurrence of fish in the main stream of Hau River was dependent not only on the down- or up-stream location but also on migration and season. The expansion or reduction in population of species in the river is a serious concern in the long term conservation. It is necessary to control the fishing according to the availability of fish in the river, especially the rare and endangered species such as Jullien's golden carp Probarbus jullieni, laotian shad Tenualosa thibaudeaui and small scaled mud carp Cirrhinus microlepis.
Acknowledgements
This study was funded by United State Geological Survey (USGS) and special thanks to Mr. Mathew E. Andersen, Senior Scientist for Biology, USGS.
Appendix
List of fishes recorded in different parts of Hau River.
References
- Baran E. 2010. Mekong fisheries and mainstream dams. Fisheries sections in: ICEM 2010. Mekong River Commission Strategic Environmental Assessment of hydropower on the Mekong mainstream, International Centre for Environmental Management, Hanoi, Viet Nam. 145 pp. [Google Scholar]
- Baran E, Baird IG, Cans G. 2005. Fisheries bioecology at the Khone Falls (Mekong river, southern Laos). Penang, Malaysia: WorldFish Center. 84p. [Google Scholar]
- Berra TM. 2001. Freshwater fish distribution. Sandiego, CA, USA: Academic Press, 604 pp. [Google Scholar]
- Cheng P, Nam S. 2011. Assessment of Gillnets and Other Fishing Gear Used in the Mekong River between Kratie and the Lao PDR Border. Inland Fisheries Research and Development Institute (IFReDI) Fisheries Administration. [Google Scholar]
- Claridge G, Sorangkhoun T, Baird IG. 1997. Community fisheries in Lao PDR: a survey of techniques and issues. IUCN–the World Conservation Union. [Google Scholar]
- Coates D, Poeu O, Suntornratnana U, Nguyen TT, Viravong S. 2005. Biodiversity and fisheries in the Mekong River Basin. In: Ohgaki S et al. (Ed.) Southeast Asian Water environment 1. IWA Publishing, 258pp. [Google Scholar]
- Deap L, Degen P, Van Zalinge N. 2003. Fishing gears of the Cambodian Mekong. Inland Fisheries Research and Development Institute of Cambodia, Phnom Penh. [Google Scholar]
- Hortle K, Suntornratana U. 2008. Socio-economics of the fisheries of the lower Songkhram River Basin, northeast Thailand, MRC, Vientiane (Lao PDR). [Google Scholar]
- Hortle KG. 2009. Chapter 9–Fisheries of the Mekong River Basin. In: Campbell IC (Ed.) The Mekong. San Diego: Academic Press, 197–249. [CrossRef] [Google Scholar]
- IUCN. 2019. The IUCN Red List of Threatened Species. Version 2019-3. http://www.iucnredlist.org. Downloaded on 10 December 2019 [Google Scholar]
- Mattson NS, Buakhamvongsa K, Sukumasavin N, Tuan N, Vibol O. 2002. Mekong giant fish species: on their management and biology. MRC Technical Paper, 29p. [Google Scholar]
- MRC (Mekong River Commission). 2017. Transboundary Fisheries Management Issues in the Mekong and Sekong Rivers. [Google Scholar]
- Nakano S-I, Tetsukazu Y, Nakashizuka T. 2012. Biodiversity Observation Network in the Asia-Pacific Region. Springer. [CrossRef] [Google Scholar]
- Ngor P, Aun S, Hortle K, Mekong River Commission V. 2006. The dai bongkong fishery for giant river prawns, Macrobrachium rosenbergii, in southeastern Cambodia. MRC Conference Series, Vientiane, Lao PDR. [Google Scholar]
- Nguyen KH. 1991. Vietnam marine fish. Part 2, Volume 1. Science and Technology Publishing house, 182 pp. (in Vietnamese) [Google Scholar]
- Nguyen ND, Smallwood C, Nguyen VH, Nguyen XT, Nguyen TT. 2006. Fishing Gears of the Mekong Delta. Research Institute for Aquaculture 2 and Mekong River Commission, Ho Chi Minh City, Viet Nam, 351pp. [Google Scholar]
- Nguyen TT, Phan TL, Nguyen KT, Truong TT, Nguyen ND, Lam NC, Vu VA, Nguyen VT, Tran AD. 2007. Report on investigation of presence of freshwater species in An Giang. Department of Science and Technology, An Giang province, 196pp. [Google Scholar]
- Pitcher TJ, Hollingworth C. 2002. Recreational fisheries: ecological, economic and social evaluation. Fish and Aquatic Resources Series, No 8. Oxford, UK: Blackwell Science, 288pp. [Google Scholar]
- Poulsen AF, Hortle K, Valbo-Jorgensen J, Chan S, Chhuon C, Viravong S, Bouakhamvongsa K, Suntornratana U, Yoorong N, Nguyen T. 2004. Distribution and ecology of some important riverine fish species of the Mekong River Basin. MRC technical paper, 10, 116pp. [Google Scholar]
- Phan TL, Pham MP, Nguyen TT, Visse T, Hortle KG. 2002. Tra Vinh Fisheries Survey − Tra Vinh Province-Viet Nam. RIA2, Department of Fisheries, Tra Vinh; Department of Statistics, Tra Vinh; AMFC of the MRC Fisheries Programme, Ho Chi Minh City, Viet Nam, 69pp + Annexes. [Google Scholar]
- Rainboth WJ. 1996. Fishes of the cambodian mekong, Food & Agriculture Org. [Google Scholar]
- Secretariat M. 1992. Fisheries in the lower Mekong basin. (Review of the fishery sector in the lower Mekong basin). Main report. Interim Mekong Committee, Bangkok. [Google Scholar]
- Tran DD, Shibukawa K, Nguyen PT, Ha HP, Tran LX, Mai HV, Utsugi K. 2013. Mô tả định loại cá Đồng Bằng Sông Cửu Long, Việt Nam. Fishes of the Mekong Delta, Vietnam. Can Tho University Publishing House, Can Tho. 174pp. [Google Scholar]
- Truong TK, Tran TTH. 1993. Identification of freshwater fish in the Mekong Delta. Faculty of Fisheries, Can Tho University, 361pp. [Google Scholar]
- Valbo-Jørgensen J, Coates D, Hortle K. 2009. Chapter 8–Fish Diversity in the Mekong River Basin. In: Campbell IC. (Ed.) The Mekong. San Diego: Academic Press, 161–196. [CrossRef] [Google Scholar]
- Vidthayanon. 2008. Field guide to fishes of the Mekong Delta. Mekong River Commission, Vientiane. [Google Scholar]
- Vuong DK. 1954–1955. Ichthyology taxonomy. Publisher of Shanghai Science, Technology and Hygiene, 843pp. (Translated by Nguyen Ba Hao) [Google Scholar]
- Welcomme R. 2001. Inland Fisheries: Ecology and Management. Food and Agriculture Organisation of the United Nations, Rome and Blackwell Science. Oxford, UK. [Google Scholar]
Cite this article as: Ut VN, Van Hoa A, Vinh HP. 2020. Status of fish biodiversity and fishing on Hau River, Mekong Delta, Vietnam. Ann. Limnol. - Int. J. Lim. 56: 14
All Tables
All Figures
 |
Fig. 1 The selected sampling sites on Hau River. |
| In the text | |
 |
Fig. 2 Morphological parameters used to measure for species identification. |
| In the text | |
 |
Fig. 3 The proportion of the total number of species by order of fish in the Hau River. A Overall, B Upper part, C Middle part, and D Lower part. |
| In the text | |
 |
Fig. 4 The Venn diagrams showing the unique and shared species between different zones (A) and fishing gears (B). |
| In the text | |
 |
Fig. 5 CPUE estimated throughout the year by trawl net in 3 sampling zones on Hau River. |
| In the text | |
 |
Fig. 6 Boxplot for the CPUE by trawl net in two seasons (dry and rainy) along Hau River. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


