| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 55, 2019
|
|
|---|---|---|
| Article Number | 22 | |
| Number of page(s) | 16 | |
| DOI | https://doi.org/10.1051/limn/2019019 | |
| Published online | 15 November 2019 | |
Research Article
Spatio-temporal distribution and species traits of water beetles along an oligotrophic hydrosystem: a case study
1
Croatian Natural History Museum, Demetrova 1, 10 000 Zagreb, Croatia
2
University of Zagreb, Faculty of Science, Department of Biology, Rooseveltov trg, 10 000 Zagreb, Croatia
3
Naturhistorisches Museum Wien, Burgring 7, 1010 Wien, Austria
4
Hrvatske vode, Central Water Management Laboratory, Ulica grada Vukovara 220, 10 000 Zagreb, Croatia
* Corresponding author: vlatkams@hpm.hr
Received:
3
April
2019
Accepted:
11
September
2019
This study presents the first comprehensive investigation of population aspects and ecological traits of water beetles in oligotrophic hydrosystems with tufa formation in southeastern Europe. Diverse lotic habitats (springs, rivers and tufa barriers) were investigated monthly for one year in Plitvice Lakes National Park in Croatia. Elmidae were the most diverse and abundant family, followed by Scirtidae and Hydraenidae. The ecological traits of water beetles were primarily defined by nutrients and water depth. Elmis bosnica Zaitzev, 1908, about which little has been published, was found to be bryophilous and to prefer low water temperatures. Biogeographical analysis revealed the dominance of typical southeastern and Mediterranean species. Species population dynamics could be attributed to differences in flow permanence, current velocity and canopy coverage. Both current velocity and water depth significantly influenced the occurrence of larval stages, while abundance of adults correlated with water depth. Our results highlight tufa barriers as preferred habitats for species of the genus Riolus and the family Scirtidae. The results of this study, many of which are the first to be reported for water beetles, provide a basis for further investigations of these animals and their ecology in oligotrophic hydrosystems with tufa. In particular, our study demonstrates the potential of Elmidae as water quality indicators, which should be considered in future conservation and protection management efforts.
Key words: biogeography / Coleoptera / Dinaric Karst / Elmis bosnica / protected area
© EDP Sciences, 2019
1 Introduction
Water beetles are completely or partially adapted to life in freshwater (Jäch, 1998). They form an ecological group that consists of numerous families. Dytiscidae and Hydrophilidae are the most speciose families, followed by Hydraenidae, Elmidae, Scirtidae, Gyrinidae, Dryopidae, and Noteridae (Jäch and Balke, 2008).
For the most part, water beetles are not cosmopolitan; instead, most species are endemic (Sánchez-Fernández et al., 2008). The Mediterranean Basin is a hotspot of water beetle biodiversity and endemism, especially lotic ecosystems in Mediterranean peninsulas (Myers et al., 2000; Griffiths et al., 2004; Jäch and Balke, 2008). The study by Picazo et al. (2012) is one of the few to investigate the taxonomic, biological and ecological traits of water beetles in Mediterranean ecosystems. Such studies are notably lacking for eastern Mediterranean lotic ecosystems around the Balkan Peninsula.
Water beetles are often used as both bioindicators and biodiversity surrogates (Abellán et al., 2005; Eyre, 2006; Sánchez-Fernández et al., 2006, 2008; Moog and Hartmann, 2017) as they inhabit numerous types of aquatic habitats (Jäch and Balke, 2008). They are sensitive, and respond quickly, to changes in environmental conditions (Eyre et al., 1990). In particular, members of the family Elmidae are widely used as indicators of water quality and, to some extent, climate change (e.g. Eyre et al., 1993; Elliott, 2008).
Substrate type, hydrogeology and current velocity are the most important abiotic factors influencing the diversity and distribution of water beetles in lotic habitats (Bournaud et al., 1992; Eyre et al., 1993). The composition and structure of aquatic vegetation also play a role (Verberk et al., 2005; Elliott, 2008; Jäch and Balke, 2008). The distribution of some families in lotic habitats is particularly influenced by longitudinal gradients of certain factors (García-Criado and Fernández-Aláez, 1995).
The present study examined water beetles of the Dinaric Karst, which extends from northeastern Italy to Albania (Bonacci, 1987; Bonacci et al., 2009). In the northeastern part of this region lies Plitvice Lakes National Park (NP) with numerous springs, tufa barriers and barrage lakes. The lakes are a sensitive ecosystem maintained by self-biodynamic processes that form the tufa. Tufa formation strongly depends on environmental conditions, particularly the level of anthropogenic impact (Srdoč, 1985).
Water beetles of the Dinaric Karst have been studied by various researchers over the past centuries (e.g. Apfelbeck, 1894; Schlosser, 1877; Novak, 1952, 1970) but these studies have been sporadic and have focused mostly on Adephaga. Recently, Mičetić Stanković et al. (2018) surveyed water beetles of River Cetina, which is the first comprehensive study of water beetle ecology in southeastern Europe. Their earlier work identified nine Elmidae species in Plitvice Lakes NP (Mičetić Stanković et al., 2015).
The water beetles in Plitvice Lakes NP have previously been studied only as a part of the benthic community (Matoničkin and Pavletić, 1967; Habdija et al., 1994, 2004). These studies concluded that the aquatic vegetation, especially bryophytes, are biodiversity hotspots in the Park and serve as “a carrier of biodiversity of lotic habitats in the NP”. They also found that the distribution of water beetles is determined by the distribution of aquatic vegetation.
The present study in lotic habitats of Plitvice Lakes NP aimed to describe the ecological traits, various population characteristics and longitudinal distribution of benthic water beetle assemblages in selected habitats. We expected to find that (i) most species would show a narrow distribution, (ii) the distribution would depend on longitudinal gradients of ecosystem characteristics, and (iii) the water beetle populations described here would show potential for assessment of water quality in lotic habitats in southeastern Europe. Additionally, we investigated in detail the biological and ecological traits of Elmis bosnica Zaitzev, 1908, which in Europe is restricted to the Balkan Peninsula and whose ecology is poorly understood (Jäch et al., 2016; Mičetić Stanković et al., 2015, 2018).
2 Material and methods
2.1 Study area
This study of benthic water beetles was conducted during the period from February 2007 to January 2008 in Plitvice Lakes NP, which has a surface area of 29,482 ha and is located in the Dinaric continental sub-ecoregion (PPNPJ, 1984). An exceptional feature of this area that has drawn international interest is the coming together of water in the form of lakes and waterfalls, together with tufa barriers that transform the river valley into a cascade of 16 picturesque barrage lakes (PPNPJ, 1984; Srdoč, 1985). This area was designated as a national park in 1949 and recognized as a UNESCO World Heritage site in 1979. The barrage lakes are supplied with water primarily from the River Matica, formed from the confluence of two short mountain rivers, Bijela rijeka and Crna rijeka. The River Matica has an average flow of 2.42 m3 s−1 and width of 7 m (Biondić and Barbalić, 2004). The barrage lake system begins with Lake Prošće, which has a surface area of 68.2 ha and maximum depth of 37.4 m, and it ends with Lake Novakovića Brod, which has a surface area of 0.40 ha and maximum depth of 4.5 m (Petrik, 1958). The lowest site represents the end of the barrage lakes and forms the beginning of the river Korana (Riđanović, 1994).
The geological bed inside the catchment area is composed of carbonates and dolomites, which form a cavernous shallow karst rich in springs and streams (Matoničkin and Pavletić, 1967). The climate alternates among moderately warm, rainy and snowy forest above 500 m a.s.l. (Makjanić, 1971/72). During the study period, annual precipitation was 1661 mm, with a minimum of 12 mm in April and maximum of 228 mm in October (Meteorological and Hydrological Institute of Croatia; www.meteo.hr). The greatest precipitation occurs during the autumn and winter months, when the air temperature can fall to −25 °C. In summer, the air temperature can be above 30 °C (Makjanić, 1971/72; Poje, 1989).
In order to investigate water beetles inhabiting “a carrier of biodiversity of lotic habitats in the Park” (Matoničkin and Pavletić, 1967; Habdija et al., 1994, 2004), the present study covered different areas of the lake system. The following 10 sites were sampled, including 12 microhabitats with aquatic vegetation (Fig. 1, Tab. 1):
-
Upper sites with rheocrene springs, and downstream sections of the small mountain rivers Bijela rijeka and Crna rijeka.
-
Tufa barriers: Labudovac, Kozjak-Milanovac and Novakovića Brod.
-
Lower sites with tufa deposits at the end of the barrage lakes: the Plitvica stream and the river Korana.
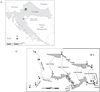 |
Fig. 1 Position of Plitvice Lakes NP in Croatia. (b) Sampling sites in Plitvice Lakes NP. 1: Spring of the Bijela rijeka (SBR), 2: Upper reach of the Bijela rijeka (URBR), 3: Spring of the Crna rijeka (SCR), 4: Middle reach of the Crna rijeka (MRCR), 5: Lower reach of the Crna rijeka (LRCR), 6: Tufa barrier Labudovac (TBLB), 7: Tufa barrier Kozjak-Milanovac (TBKM), 8: Tufa barrier Novakovića Brod (TBNB), 9: River Korana in village of Korana (RK), 10: Plitvica stream (PS). *Sites from 6 to 8 are also presented in lateral profile because they are located at barriers between adjacent lakes. |
Characteristics of the sampling sites in Plitvice Lakes National Park. Abbreviations: SBR: Spring of the Bijela rijeka, URBR: Upper reach of the Bijela rijeka, SCR: Spring of the Crna rijeka, MRCR: Middle reach of the Crna rijeka, LRCR: Lower reach of the Crna rijeka, TBLB: Tufa barrier Labudovac, TBKM: Tufa barrier Kozjak-Milanovac, TBNB: Tufa barrier Novakovića Brod, PS: Plitvica stream, RK: river Korana in the village of Korana; A: angiosperms, B: bryophytes, p: pebbles, s: stones, tc: tufa chunks.
2.1.1 Sampling and identification of water beetles
Benthic water beetles were sampled monthly during the study period using a Surber sampler (25 × 25 cm) in all microhabitats with aquatic vegetation, except at the Lower reach of the Crna rijeka, where greater water depth required the use of a hand net. The same areas were sampled every month. The Surber and hand nets had a mesh size of 500 μm. Altogether 144 samples were analyzed, and the number of individuals was calculated for an area of 1 m2. Specimens were identified to the lowest possible taxonomic level using the following literature: Janssens (1965), Olmi (1976), Berthélemy (1979), Franciscolo (1979), Jäch and Brojer (2006), Pirisinu (1981). When necessary, specimens were identified by comparison with specimens held in the World Water Beetle Collection and Research Centre at the Natural History Museum in Vienna. Females of Elmis (Elmidae) and Helophorus (Helophoridae) could not be identified to species level, nor could most larvae. Voucher specimens of water beetles are housed in the Croatian Natural History Museum.
2.1.2 Abiotic and biotic environmental parameters
Abiotic and biotic parameters were analyzed at all 10 sites, always at the same location within the site and at the same time of day. The following physical and chemical properties of water were measured: pH (with pH meter WTW pH 330), water temperature, dissolved oxygen concentration and oxygen saturation (with WTW Oxi 330/SET oximeter), alkalinity (by titration with 0.1 M HCl), conductivity (with WTW LF 330 conductivity meter), current velocity (with P-670-M velocimeter), water depth (with hand-held meter) and nutrients [ammonium, by HRN ISO 70-3:1998 method; nitrate plus nitrite nitrogen (NO3-NO2), by HRN ISO 7890-3:2001 method; and orthophosphates, by HRN ISO 6878:2001 method]. Aquatic vegetation was identified according to the following literature for identification of aquatic bryophytes and aquatic angiosperms: Atherton et al. (2010), Casper and Krausch (2008a,b), Frahm and Frey (2004), Frey et al. (2006), Pavletić (1968), Smith (2004). For each sampling site, the geographical coordinates were recorded using a GPS (Garmin Oregon 550) and processed in ArcGIS software.
2.1.3 Data analysis
Traits of benthic water beetles in Plitvice Lakes NP were analyzed in terms of (1) population aspects [species richness, abundance, seasonal dynamics and adult/larvae ratio (Ad/Lv)], (2) ecological aspects (biogeography and preferences for current velocity, water temperature, pH, alkalinity, oxygen, nutrients, conductivity, water depth and vegetation type), and (3) longitudinal distribution as described by Moog and Hartmann (2017). The percentage of water beetle abundance was calculated for each biocoenotic zone (Crenon-Rhithron-Potamon Concept). Biogeographical analysis was conducted according to Nilsson (2003, 2015), Fikáček et al. (2015), Jäch and Skale (2015), and Jäch et al. (2016), while zoogeographical categorization was conducted according to Vigna Taglianti et al. (1999) and Darilmaz et al. (2012). Species richness was calculated for each microhabitat. Pairwise dissimilarity between microhabitats was measured using non-metric multidimensional scaling analysis (NMDS) based on a Bray-Curtis similarity matrix (Zuur et al., 2007). A general linear model (GLM) was used to test the significance of variation among the sampling sites, classified as lower sites (KR, PS), barriers (TBNB, TBLB, TBKM), upper sites (MRCR, LRCR, URBR) and springs (SBR, SCR). Month was used as a random factor due to temporal pseudo-replication (Hurlbert, 1984), and multiple comparisons were performed using the post hoc Scheffe test.
Analyses were conducted using water beetle assemblages as the dependent variable and physical and chemical properties of water as independent variables. On the microhabitat scale, relationships of environmental factors (water velocity and depth) to annual mean density of water beetles were analyzed using Spearman's rank correlation coefficient (ρ). This analysis was performed separately for adults and for larvae. Prior to the correlation analysis, normality tests were performed for all data using the D'Agostino's K-squared test and the Kolmogorov-Smirnov (K-S) test. Canonical correspondence analysis (CCA) was used to estimate response of water beetle abundances to environmental variables, and results were assessed for significance using the Monte Carlo permutation test (with 999 permutations). Abundances of both larvae and adults were used. Biological data were log-transformed prior to analysis, while physical and chemical data were normalized. Analyses involved approximately 22 species and nine physical and chemical parameters at 12 microhabitats. A full draftsman's plot excluded orthophosphates and nitrites. Analyses were performed using SPSS 17.0 (SPSS Statistics, 2008) and CANOCO for windows software package ver. 5.0 (Ter Braak and Šmilauer, 2012). Figures were created using Adobe® Illustrator® CS6.
3 Results
3.1 Faunal assemblages and longitudinal distribution
A total of 13,675 individuals were collected belonging to seven families, 13 genera, and at least 22 species, 19 of which were named. Overall, the family Elmidae was the most diverse family with nine species (Tab. 2).
The lowest number of species was recorded in the spring of the Crna rijeka (SCR). The highest number of species was recorded in the Plitvica stream (PS). Elmidae and Scirtidae were most abundant, while Dytiscidae and Gyrinidae were least abundant. Elmis bosnica was dominant in all microhabitats of the upper sites, except at the lower reach of Crna rijeka, where Hydraena melas was dominant (Tab. 2). Water beetles were more abundant at tufa barriers than at all the other sites sampled. The most abundant water beetles were Riolus subviolaceus, R. cupreus and larvae of the family Scirtidae.
The highest number of water beetle species was found during four months (February, March, June, and July), while the lowest number was encountered in January. During all months of the study, we detected six elmid species; Hydrocyphon deflexicollis (Müller, 1821), which we identified based on adult specimens caught in the emergence traps positioned in sampled habitats; and other unidentified individuals of the family Scirtidae. Scirtidae pupae and five species (e.g. Laccobius striatulus and Oulimnius tuberculatus) were found only in one month (Tab. 3). Overall, the highest number of individuals was recorded at tufa barriers during the winter months (Fig. 2).
The Ad/Lv ratio differed greatly among species (Tab. 2), though it consistently showed a bias in favor of larvae (Fig. 2), especially among Elmidae (Limnius volckmari Ad/Lv = 0.113, Riolus species Ad/Lv = 0.286, Esolus parallelepipedus Ad/Lv = 0.074). Pupal stages of families Elmidae and Scirtidae were also found. Elmidae pupae were recorded during four months (January, June, August and November), while Scirtidae pupae were recorded only in June (Tabs. 2 and 3).
In the NMDS plot with a stress value of 0.09, microhabitats clustered into two groups: one contained upper sites, while the other contained the tufa barriers, Plitvica stream and Korana rijeka (Fig. 3). MGLM confirmed these results, showing significant differences between microhabitats at the barriers (TBKM, TBLB, TBNB) and microhabitats in the Bijela rijeka and Crna rijeka (barriers − springs: p = 0.007; barriers − upper sites: p = 0.002; p ≤ 0.05).
The longitudinal distribution of benthic water beetles in Plitvice Lakes NP is shown in Figure 4. The highest proportion of crenal species was recorded in springs, while littoral species reached their highest proportion in the Bijela rijeka. Along the length of the Crna rijeka, the proportion of crenal species decreased while the proportion of rhithral and potamal species increased. Rhithral species made up the highest proportion at the other sites sampled.
Taxonomic list, abundance, species richness and distribution of larval and adult stages of water beetles in Plitvice Lakes NP from February 2007 to January 2008. Abbreviations: Lv.: larval stage, Ad.: adult stage; S2: microhabitat with angiosperms; S3: microhabitat with bryophytes. Sampling sites are abbreviated as in Table 1.
Seasonal dynamics of water beetles at 12 microhabitats from February 2007 to January 2008 in Plitvice Lakes NP.
 |
Fig. 2 Temporal variation in abundance of water beetles and overall adult/larvae ratio from February 2007 to January 2008 in Plitvice Lakes NP. |
 |
Fig. 3 NMDS analysis of microhabitats in Plitvice Lakes NP from February 2007 to January 2008. Abbreviations: S2: microhabitat with angiosperms; S3: microhabitat with bryophytes. For locality abbreviations, see Figure 1. |
 |
Fig. 4 Longitudinal distribution of water beetles in Plitvice Lakes NP. Abbreviations of biocoenotic regions: EUC: Eucrenal, HYC: Hypocrenal, ER: Epirhithral, MR: Metarhithral, HR: Hyporhithral, EP: Epipotamal, MP: Metapotamal, LT: Littoral. For locality abbreviations, see Figure 1. |
3.2 Biogeographical analysis of water beetles and population dynamics of Elmis bosnica
The biogeography of the identified benthic water beetles in Plitvice Lakes NP is shown in Figure 5. Biogeographical analysis showed dominance of the European chorotype in water beetle assemblages, which comprised mainly typical southeastern and Mediterranean species. The lowest proportion was recorded for Balkan chorotypes and widespread species.
The phenology and Ad/Lv ratio of Elmis bosnica in the Bijela rijeka and Crna rijeka are presented in Figure 6. The Ad/Lv ratio of the species differed greatly among the microhabitats of these rivers. The difference was especially evident when only spring microhabitats with bryophytes were compared. From August to October and from May to July, larvae were considerably more abundant than adults at sampling site SCR S3, while the converse was true at sampling site SBR S3.
Spatial variation in abundance was recorded both for larvae and adults of Elmis bosnica in the Bijela rijeka. Adults were more numerous in bryophytes than angiosperms at all microhabitats. The abundance of both life stages decreased along the length of the Crna rijeka. Larval stages were more frequent there than adults, especially at the downstream site LRCR, where only larvae occurred during four months of the study (April, May, June and November) (Tab. 2, Fig. 6).
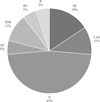 |
Fig. 5 Biogeography of identified water beetles in Plitvice Lakes NP. Abbreviations of biogeographical chorotypes: AE: Asiatic; B: Balkan; BT: Balkano-Turanian; CAE: Centralasiatic-European; E: European; TE: Turano-European; TEM: Turano-Europeo-Mediterranean. |
 |
Fig. 6 Seasonal dynamics and adult/larvae ratio of Elmis bosnica in the Bijela rijeka and the Crna rijeka from February 2007 to January 2008 at: a) springs and b) along the watercourse. Abbreviations: Ad.: adult stage, Lv.: larval stage; S2: microhabitat with angiosperms; S3: microhabitat with bryophytes. For locality abbreviations, see Figure 1. |
3.3 Environmental characteristics and water beetles
The aquatic vegetation sampled in the microhabitats included bryophytes (seven species) and angiosperms (two species) (Tab. 2). The highest species richness of plants was recorded at the tufa barrier Kozjak-Milanovac.
Elmis bosnica had its highest abundance in the bryophyte community consisting of Cinclidotus aquaticus and Platyhypnidium riparoides. Limnius volckmari preferred the angiosperm Apium repens. Hydraena melas was recorded in both bryophytes and angiosperms. All three species of Riolus were most abundant in a community that consisted of four bryophyte species. Individuals of the family Scirtidae were most abundant in communities of Hygroamblystegium fluviatile and H. tenax var. tenax (Tabs. 1 and 2).
The ranges of measured environmental variables are presented in Table 1. GLM analysis indicated significant differences among sampled sites in the following physical and chemical properties of water: water temperature (barriers − springs: p ≤ 0.0005, barriers − upper sites: p ≤ 0.0005, upper sites − lower sites: p = 0 0.005, p ≤ 0.05), oxygen concentration (springs − upper sites: p = 0.022, p ≤ 0.05), oxygen saturation (barriers − springs: p = 0.002, p ≤ 0.05, springs − lower sites: p ≤ 0.0005), pH (barriers − springs: p ≤ 0.0005, barriers − upper sites: p = 0.002, p ≤ 0.05, springs − lower sites: p ≤ 0.0005), conductivity (barriers − springs: p ≤ 0.0005, barriers − upper sites: p ≤ 0.0005, springs − lower sites: p ≤ 0.0005, springs − upper sites: p ≤ 0.0005), alkalinity (barriers − springs: p ≤ 0.0005, barriers − upper sites: p ≤ 0.0005, springs − lower sites: p = 0.001, p ≤ 0.01, upper sites − lower sites: p ≤ 0.0005), nitrates (barriers − springs: p ≤ 0.0005, barriers − lower sites: p = 0.055, p ≤ 0.05, barriers − upper sites: p ≤ 0.0005), velocity (barriers − springs: 0.0028, p ≤ 0.01, barriers − upper sites: 0.009, p ≤ 0.01) and water depth (barriers − upper sites: 0.025, p ≤ 0.05). Based on Spearman's correlation coefficient, Hydraena melas and H. subintegra annual mean density correlated positively with current velocity at microhabitat URBR S2 (ρ = 0.651, p = 0.041, N = 10; p ≤ 0.05). Scirtidae correlated positively with water depth at SBR S2 (ρ = 0.711, p = 0.021, N = 10; p ≤ 0.05) and URBR S2 (ρ = 0.793, p = 0.006; p ≤ 0.01, N = 10) but negatively at TBKM S3 (ρ= −0.655, N = 10; p ≤ 0.05). Larval annual mean density correlated negatively with water depth for Elmis bosnica at URBR S3 (ρ = −0.722, p = 0.018, N = 10; p ≤ 0.05) as well as Riolus subviolaceus and R. cupreus at TBKM S3 (ρ = −0.754, p = 0.012, N = 10; p ≤ 0.05).
CCA eigenvalues for the first two axes were 0.541 and 0.157, accounting for 83.1% of the variance in the species-environment relationship. The Monte Carlo permutation test showed that the ordination was significant (first axis: F-ratio = 18.212, p = 0.0020; overall: trace = 0.839, F-ratio = 3.419, p = 0.0020). The first canonical axis strongly correlated with nitrates (R 1 = 0.6979) and ammonium ions (R 1 = 0.6923). Axis 2 showed the highest correlation with water depth (R 2 = 0.4143). Based on the ordination plot (Fig. 7), Oulimnius tuberculatus, Esolus parallelepipedus and all three species of Riolus showed the strongest correlation with current velocity, water temperature and oxygen saturation. Hydraena melas, H. subintegra, H. riparia, Elmis bosnica and Limnius volckmari showed the strongest correlation with conductivity and water depth. Alkalinity was the most significant factor for Elmis aenea, E. rioloides, Ochthebius metallescens, Laccobius striatulus and Scirtidae.
 |
Fig. 7 Canonical correspondence analysis of water beetles in Plitvice Lakes NP. Abbreviations: Elm_a_r: Elmis aenea/rioloides, Lim_vol_sp: Limnius volckmari/sp., Riol_cup_sub: Riolus cupreus/subviolaceus. Other taxa are abbreviated as in Table 2. |
4 Discussion
Water beetles are one of the most studied insect groups in the western Mediterranean, especially in southeast Spain (e.g. Ribera, 2000; Abellán et al., 2005; Sánchez-Fernández et al., 2006, 2008). We are unaware of similar studies of water beetles in the eastern Mediterranean, with the exception of the work by Darilmaz et al. (2012) on zoogeography of water beetles in northern Turkey. The present study presents the first comprehensive investigation of population aspects and ecological traits of water beetles in oligotrophic hydrosystems with tufa formation in southeastern Europe. The results of this study, many of which are the first to be reported for water beetles, can help guide further investigations of these animals and their ecology in oligotrophic hydrosystems in the eastern Mediterranean.
4.1 Faunal assemblages and longitudinal distribution
Previous investigations of the water beetles in Plitvice Lakes NP were conducted sporadically (Matoničkin and Pavletić, 1967; Matoničkin et al., 1971), and the present study identified 11 species not previously reported in the NP. In this area, species richness of water beetles appears to be as high as that of other benthic insects (see Kučinić and Malicky, 2002; Popijač and Sivec, 2009; Ivković et al., 2010, 2012, 2014; Previšić et al., 2010, 2013; Vilenica et al., 2014).
The genus Elmis dominated at all sites sampled, except in the lower reach of the Crna rijeka, where the slower current and dense angiosperms favor the detritophilous Hydraena melas (Jäch et al., 2005). We detected only E. bosnica in the spring areas of the Bijela rijeka and Crna rijeka, in contrast to a previous study of freshwater habitats in Plitvice Lakes NP (Matoničkin and Pavletić, 1967), which detected Elmis maugetii in the spring areas of both rivers. This earlier result is therefore doubtful (see Mičetić Stanković et al., 2015), especially since these two species are difficult to distinguish. Furthermore, the present study revealed a wider distribution of the genus Hydraena throughout Plitvice Lakes NP than previously recorded (Matoničkin and Pavletić, 1967; Matoničkin et al., 1971).
The distribution of benthic water beetles along a river follows a longitudinal gradient, reflecting the changes in habitat (Berthélemy, 1964; Brojer et al., 2017). The present results showed the same tendency: the species richness of water beetles increased with distance from the spring area. Species with a narrow distribution range prefer upper sites, while widely distributed species, especially of the European chorotype, prefer lower sites and tufa barriers. Our results are consistent with the idea that the genus Elmis is a good indicator of stream zonation, as suggested by Bournaud et al. (1992): we found Elmis bosnica only in upper sites, in accordance with the hypothesis that it is the only Elmis species in springs and spring areas of the Dinaric Karst (Mičetić Stanković et al., 2015, 2018). Conversely, spring areas in Austria and Central Europe are inhabited with three Elmis species: E. aenea, E. latreillei (Bedel, 1878) and E. rietscheli Steffan, 1958 (Brojer et al., 2017). E. aenea and E. rioloides show similar traits both in the Plitvice Lakes NP (present study) and in the River Cetina (Mičetić Stanković et al., 2018). The highest abundance of these lotic species was recorded at high-flow sites distant from spring areas. The rhithral features of both species have also been noted in rivers and streams in other parts of Europe (e.g. Olmi, 1976; Hebauer, 1994a,b; Eyre et al., 2006).
Most water beetle species in Plitvice Lakes show a narrow distribution, and are present elsewhere in southeastern Europe and the Middle East (Nilsson, 2003, 2015; Fikáček et al., 2015; Jäch and Skale, 2015; Jäch et al., 2016). This is consistent with Croatia's position in southern Europe.
4.2 Temporal dynamics of the Ad/Lv ratio and Elmis bosnica abundance
Previous studies showed that water beetle species richness increases in the summer and decreases during the winter (Nilsson, 1996; Giller and Malmqvist, 1998). The present study showed seasonality of abundance and species richness, but the two variables peaked at different periods of the year. Species composition caused seasonal dynamics to differ between adults and larvae. For instance, Hydraenidae lay their eggs in spring and early summer (Hansen, 1996), which could contribute to the observed peak in species richness in March. The fact that Hydraenidae are mostly terrestrial, at the larval stage (Olmi, 1976; Hansen, 1996), may contribute to the observed lower overall species richness in winter. The results of the present study confirmed that the life stages of Elmidae overlap during a one-year cycle (Berthélemy and De Riols, 1965; Dietrich and Waringer, 1999; Elliott, 2006). Additionally, the present study found that Elmis adults were most abundant from May to August, while Elmis larvae dominated in autumn and winter, which concurs with studies by Berthélemy and Ductor (1965) and by Dietrich and Waringer (1999).
The seasonal dynamics of Limnius volckmari in the present study are similar to those described by Maitland (1967). Nilsson (1996) found that adults of Elmidae emerged in early summer after the pupation of overwintering larvae, and that oviposition took place in summer, such that higher numbers of small larvae were recorded during late summer and early autumn.
Our study found different seasonal dynamics for specimens of the family Scirtidae between the upper sites and other sites sampled. The Scirtidae recorded from the upper sites (mainly Elodes) show the same winter-only presence as described by Cuppen (1993). At other sites in our study, Scirtidae (mainly Hydrocyphon deflexicollis) were present throughout the year. This difference may reflect warmer water temperatures and/or more plentiful food in downstream areas than in organically depleted spring areas, which should be explored in further studies. The stably favorable conditions in downstream areas may support development of numerous scraper and collector-gatherer larvae of this family.
Matoničkin and Pavletić (1967) recorded a significant decrease in the number of adults of Elmis bosnica, which they misidentified as “E. maugetii”, during the winter months in the Crna rijeka, which was also the case in the present study. In the Bijela rijeka, in contrast, this species was detected throughout the year. This difference, which has also been reported for Diptera at the same sites (Ivković et al., 2015), may be due to differences in flow permanence and current velocity during the study period. For instance, flow permanence and current velocity varied more in the spring of Crna rijeka than in the spring of the Bijela rijeka. Furthermore, the spring of the Crna rijeka is shaded by dense coniferous forest, while the spring of the Bijela rijeka is unshaded, with dense aquatic vegetation (Ivković et al., 2015). The spring of the Bijela rijeka thus appears to provide conditions more favorable for water beetles than the spring of the Crna rijeka. Indeed, Giller and Malmqvist (1998) and Erman (2002) stressed canopy coverage as a major environmental determinant of the composition of benthic communities in rivers and streams. The present study showed that Elmis bosnica adults prefer bryophytes, which are preferred by other Elmis species as well (Berthélemy, 1966).
4.3 Environmental characteristics and water beetles
Comparison of the physico-chemical characteristics of the water in Plitvice Lakes NP between the present study and the 1960s (Petrik, 1961; Matoničkin and Pavletić, 1967) confirms an increase in anthropogenic pressure in the Park. Those studies in the 1960s already showed that tourism had led to nutrient enrichment (ammonium ion concentration) in the NP, especially in the Crna rijeka. The results of the present study highlight the substantial increase in anthropogenic pressure on the NP through tourism, reinforcing the need to balance economic growth with sustainability (Mužinić and Filipović, 2006).
Elodes sp. showed a distribution pattern throughout the Plitvice Lakes NP similar to that of Elmis bosnica, while Hydrocyphon deflexicollis showed a distribution similar to that of larvae of Riolus subviolaceus and R. cupreus. These habitat preferences may reflect larval morphology (Elliott, 2008). Elodes sp. and larvae of E. bosnica have broadly flattened bodies and prefer habitats in upper sites with abundant moss cover on a solid riverbed, in contrast to H. deflexicollis and Riolus species, which have a cylindrical body shape and prefer tufa barriers with tufa chunks and associated mosses.
Vegetation type and structure highly influenced water beetle assemblages throughout the Park, as angiosperms increase beetle species richness more than bryophytes do. Angiosperms increase habitat complexity and therefore the diversity of benthic invertebrates (Giller and Malmqvist, 1998, Mičetić Stanković et al., 2018). Our finding of high abundance of water beetles at the tufa barriers, especially Kozjak-Milanovac, where aquatic vegetation was also most diverse (four bryophyte species), may be explained by the water quality as well as by dense and diverse moss cover. The water that pours over the tufa barrier from Lake Kozjak to Lake Milanovac is organically enriched because it originates in the epilimnion layer. This provides favorable conditions for the development of a rich phytobenthos layer on the surface of bryophytes. Indeed, Plenković-Moraj et al. (2002) already reported the dominance of diatoms (Bacillariophyceae) in the tufa barriers in Plitvice Lakes NP. This environment should also provide optimal conditions for scraper and collector-gatherer Riolus species, since diatoms are the preferred food of Riolus subviolaceus (Beier, 1948; Jäch, 1982). This likely explains why R. subviolaceus and R. cupreus dominated in the tufa barriers in the present study.
Esolus and Limnius in our study preferred vegetation growing on riverbeds formed of coarse sediment particles over vegetation growing on riverbeds formed of solid stones. This contrasts with the preference of Elmis, which is consistent with the study by Elliott (2008).
Our analysis confirms several previously reported water beetle habitat preferences, such as the preference of Elmis aenea for alkaline water (Eyre et al., 1993; Mičetić Stanković et al., 2018). It also confirms published preferences of Esolus parallelepipedus, Riolus subviolaceus and R. cupreus for high oxygen content and fast current (Bournaud et al., 1992; Hebauer, 1994a; Dietrich and Waringer, 1999). It supports the eurytopic character of E. parallelpipedus (García-Criado et al., 1999), which we detected at upper sites, tufa barriers and lowest sites. The published preference of R. subviolaceus and Hydraena minutissima for calcareous substratum (García-Criado et al., 1999) likely explains why these species were absent from upper sites without tufa deposits in our study. Hydraena gracilis preferred high alkalinity in our study, in agreement with work by Eyre et al. (1993). In our study, Hydraena minutissima was recorded only in substrates with bryophytes, consistent with its preference for mountain springs, rivers and streams, and the presence of Fontinalis bryophytes (Pirisinu, 1981; Hebauer, 1994a; Klausnitzer, 1996). Hydraena riparia, which is widely distributed throughout the Palearctic Region (Jäch and Skale, 2015), showed a preference for moderate current velocity and angiosperms in our study, consistent with results from Bournaud et al. (1992). Helophorus brevipalpis strongly preferred angiosperms in the riparian zone in our study, consistent with other work (Bournaud et al., 1992; Eyre et al., 1993; Hebauer, 1994a) but in contrast to one analysis suggesting a eurytopic character for this species (Foster et al., 2014).
While supporting numerous aspects of the literature on water beetle habitat preferences, our study also substantially extends the literature with some first insights. Our results suggest that Elmis aenea and E. rioloides prefer shallower water depth, and that E. bosnica is bryophilous and prefers low water temperatures (Mičetić Stanković et al., 2015, 2018). Our results further suggest that the poorly studied Hydraena subintegra, endemic to the Balkan Peninsula (Jäch and Skale, 2015), prefers deeper water with higher conductivity and slower current. Individuals of the family Scirtidae in Plitvice Lakes NP appear to favor deeper, alkaline water and fast current, consistent with previous studies (Bournaud et al., 1992; Cuppen, 1993). Elodes sp. shows a preference for mountainous lotic habitats, especially the Bijela rijeka, which has an open canopy (Ivković et al., 2015). On the contrary, Hydrocyphon deflexicollis seems to prefer tufa barriers, which have a cavernous structure and fast current. Our study suggests that the distribution of Limnius volckmari can depend on water depth and conductivity, in contrast to the suggestion of Maitland (1967). Further studies should explore whether this discrepancy is true or reflects the influence of other variables, such as biotic interactions.
In conclusion, the present study is the first comprehensive analysis of population aspects and ecological traits of water beetles in specific karst habitats. Benthic water beetles, especially Elmidae, may be useful as environmental descriptors because their composition and distribution are strongly influenced by nutrients and water depth, as well as other abiotic and biotic factors. The extreme sensitivity of Elmis bosnica to environmental conditions means that this species should be included in conservation and protection programs at spring areas in southeastern Europe. Permanent conservation programs are needed to protect these unique habitats.
Acknowledgements
This comprehensive study in Plitvice Lakes NP was conducted with the permission of the Ministry of Environmental and Nature Protection of the Republic of Croatia (RN: 532-08-02-1/7-06-3; 532-08-02-1/7-06-3). The authors would like to thank Andreja Komljenović, Martina Krbavčić, Sanja Žalac, Bruno Polak, Matija Šimac and Prof. Zlatko Mihaljević for their indispensable assistance during the field investigations. Many thanks are due to Mr. Ivančica Krulik, Mirjana Jelenčić and Sanja Sviben for help with sorting the collected material. We are grateful to Michaela Brojer (Naturhistorisches Museum Wien, Austria) for assistance with the identification of certain specimens, and to Prof. Antun Alegro for help with the identification of aquatic vegetation. We thank Dr. Gabor Várbíró and Dr. Debarshi Dey for help in statistical analysis. We thank Dr. Anita Belančić for literature loan. We are very grateful to Dr. Adrian C. Pont (Oxford University Museum of Natural History, UK) and Dr. Bradley Sinclair (Canadian National Collection of Insects and Ottawa Plant Laboratory − Entomology Canadian Food Inspection Agency) for proofreading the manuscript. The study is a result of projects No. 119-1193080-1206 (PL: M. Kučinić) and No. 119-1193080-3076 (PL: M. Kerovec) supported by the Croatian Ministry of Science, Education and Sports.
References
- Abellán P, Sánchez-Fernández D, Velasco J. Millán A. 2005. Assessing conservation priorities for insects: status of water beetles in south Spain. Biol Conserv 121: 79–90. [CrossRef] [Google Scholar]
- Apfelbeck V. 1894. Fauna insectorum Balcanica. Beiträge zur Kenntnis der Balkanfauna 2. Wissenschaftliche Mitteilungen aus Bosnien und der Herzegowina, pp. 24–32. [Google Scholar]
- Atherton I, Bosanquet S, Lawley M. 2010. Mosses and liverworts of Britain and Ireland − a field guide. United Kingdom, Plymouth: British Bryological Society, 848 pp. [Google Scholar]
- Beier M. 1948. Zur Kenntnis von Körperbau und Lebensweise der Helminen (Col. Dryopidae). «EOS». Rev Esp Entomol 24: 123–211. [Google Scholar]
- Berthélemy C. 1964. La zonation des Plécoptères et des Coléoptères dans les cours d'eau des Pyrénées. Gewässer und Abwässer 34/35: 77–79. [Google Scholar]
- Berthélemy C. 1966. Recherches écologique et biogéographiques sur le Plécoptères et des Coléoptères d'eau courante (Hydraena et Elminthidae) des Pyrénées. Ann Limnol 2: 227–458. [CrossRef] [Google Scholar]
- Berthélemy C. 1979. Elmidae de la région paléarctique occidentale: systématique et répartition (Coleoptera Dryopoidea). Ann Limnol 15: 1–102. [CrossRef] [Google Scholar]
- Berthélemy C, Ductor M. 1965. Taxonomie larvaire et cycle biologique de six espèces d'Esolus et d'Oulimnius européens (Coleoptera Dryopoidea). Ann Limnol 1: 257–276. [CrossRef] [Google Scholar]
- Berthélemy C, De Riols J. 1965. Les larves ďElmis du groupe ďE. maugetii (Coléoptères Drypoidea). Ann Limnol 1: 21–38. [CrossRef] [Google Scholar]
- Biondić D, Barbalić D. 2004. Maksimalni protoci hrvatskih vodotoka crnomorskog sliva. In: Žugaj R. (Ed.), Seminar Velike i male vode, Zbornik radova, Hrvatsko hidrološko društvo, Zagreb, pp. 27–40 [In Croatian]. [Google Scholar]
- Bonacci O. 1987. Karst hydrology and water resources − past, present and future. In: Rodda JC, Matalas NC (Eds.), Water for the future: Hydrology in Perspective, Proceedings of the Rome Symposium, April 1987, IAHS Publ., Rome, pp. 205–213. [Google Scholar]
- Bonacci O, Pipan T, Culver DC. 2009. A framework for karst ecohydrology. Environ Geol 56: 891–900. [CrossRef] [Google Scholar]
- Bournaud M, Richoux P, Usseglio-Polatera P. 1992. An approach to the synthesis of qualitative ecological information from aquatic Coleoptera communities. Regul Rivers Res Mgmt 7: 165–180. [CrossRef] [Google Scholar]
- Brojer M, Jäch MA, Kodada J, Moog O. 2017. COLEOPTERA: Water Beetles s.l. In: Moog O, Hartmann A. (Eds.), Fauna Aquatica Austriaca, 3. Edition 2017. – Wien: Federal Ministry of Agriculture, Forestry, Environment and Water Management. http://www.ecoprof.at/index.php/faunaaquaticaaustriaca.html [Google Scholar]
- Casper SJ, Krausch HD. 2008a. Süβwasserflora von Mitteleuropa 24: Pteridophyta und Anthophyta 1. Teil: Lycopodiaceae bis Orchidaceae. Heidelberg: Spektrum Akademischer Verlag, 404 pp. [Google Scholar]
- Casper SJ, Krausch HD. 2008b. Süβwasserflora von Mitteleuropa 24: Pteridophyta und Anthophyta 2. Teil: Saururaceae bis Asteraceae. Heidelberg: Spektrum Akademischer Verlag, 942 pp. [Google Scholar]
- Cuppen JGM. 1993. Flight periods of Scirtidae (Coleoptera) based on weekly samples from a malaise trap. Entomol Ber 53: 137–142. [Google Scholar]
- Darilmaz MC, Jäch MA, Skale A. 2012. Biodiversity and zoogeography of water beetles from the Kemaliye, Northern Turkey (Coleoptera). Spixiana 35: 101–108. [Google Scholar]
- Dietrich F, Waringer JA. 1999. Distribution patterns and habitat characterization of Elmidae and Hydraenidae (Insecta: Coleoptera) in the Weidlingbach near Vienna, Austria. Int Rev Hydrobiol 84: 1–15. [Google Scholar]
- Elliott JM. 2006. Critical periods in the life cycle and the effects of a severe spate vary markedly between four species of elmid beetles in a small stream. Freshw Biol 51: 1527–1542. [CrossRef] [Google Scholar]
- Elliott JM. 2008. The ecology of riffle beetles (Coleoptera: Elmidae). Freshw Biol 1: 189–203. [Google Scholar]
- Erman NA. 2002. Lessons from a long-term study of springs and spring invertebrates (Sierra Nevada, California, U.S.A) and implications for conservation and management. In: Sada DW, Sharpe SE (Eds.), Proceedings of the meeting on spring-fed wetlands: important scientific and cultural resources of the intermountain region, May 2002, Las Vegas, Nevada. Reno: Desert Research Institute, 1–13. [Google Scholar]
- Eyre MD. 2006. A strategic interpretation of beetle (Coleoptera) assemblages, biotopes, habitats and distribution, and the conservation implications. J Insect Conserv 10: 151–160. [CrossRef] [Google Scholar]
- Eyre MD, Foster GN, Foster AP. 1990. Factors affecting the distribution of water beetle species assemblages in drains of eastern England. J Appl Entomol 109: 217–225. [CrossRef] [Google Scholar]
- Eyre MD, Foster GN, Luff ML, Rushton SP. 2006. The definition of British water beetle species pools (Coleoptera) and their relationship to altitude, temperature, precipitation and land cover variables. Hydrobiologia 560: 121–131. [CrossRef] [Google Scholar]
- Eyre MD, Pilkington JG, Carr R, McBlane RP, Rushton SP, Foster GN. 1993. The running-water beetles (Coleoptera) of a river catchment in northern England. Hydrobiologia 264: 33–45. [CrossRef] [Google Scholar]
- Fikáček M, Angus RB, Gentili E, et al. 2015. Helophoridae, Hydrochidae, Hydrophilidae. In: Löbl I, Löbl D. (Eds.), Catalogue of Palaearctic Coleoptera, Vol. 2. Hydrophiloidea − Staphylinoidea, Revised and updated edition. Brill: Leiden, pp. 25–76. [Google Scholar]
- Foster GN, Bilton DT, Friday LE. 2014. Keys to adults of the water beetles of Britain and Ireland (Part 2) (Coleoptera: Polyphaga: Hydrophiloidea − both aquatic and terrestrial species). In: Wilson M (Ed.), Handbooks for the identification of British Insects, Vol 4, Part 5b. St. Albans: Royal Entomological Society, 126 pp. [Google Scholar]
- Frahm J-P, Frey W. 2004. Moosflora, 4th edn. Stuttgart: Eugen Ulmer Verlag, 538 pp. [Google Scholar]
- Franciscolo EM. 1979. Fauna d'Italia. Vol. XIV. Coleoptera Haliplidae, Hygrobiidae, Gyrinidae, Dytiscidae. Bologna: Calderini, 804 pp. [Google Scholar]
- Frey W, Frahm JP, Fischer E, Lobin W, Blockeel TL. 2006. The liverworts, mosses and ferns of Europe. Colchester: Harley Books, 527 pp. [Google Scholar]
- García-Criado F, Fernández-Aláez M. 1995. Aquatic Coleoptera (Hydraenidae and Elmidae) as indicators of the chemical characteristics of water in the Orbigo River basin (N-W Spain). Ann Limnol 31: 185–199. [CrossRef] [Google Scholar]
- García-Criado F, Fernández-Aláez C, Fernández-Aláez M. 1999. Environmental variables influencing the distribution of Hydraenidae and Elmidae assemblages (Coleoptera) in a moderately-polluted river basin in north-western Spain. Eur J Entomol 96: 37–44. [Google Scholar]
- Giller PS. Malmqvist B. 1998. The biology of streams and rivers. Oxford: Oxford University Press, 304 pp. [Google Scholar]
- Griffiths HI, Kryštufek B, Reed JM. 2004. Balkan biodiversity: pattern and process in the European hotspot. Dordrecht: Oxford University Press, 191 pp. [Google Scholar]
- Habdija I, Primc-Habdija B, Belinić I. 1994. Functional community organization of macroinvertebrates in lotic habitats of the Plitvice Lakes. Acta Hydrochim Hydrobiol 22: 85–92. [CrossRef] [Google Scholar]
- Habdija I, Primc-Habdija B, Matoničkin R, et al. 2004. Current velocity and food supply as factors affecting the composition of macroinvertebrates in bryophyte habitats in karst running water. Biologia (Bratisl.) 59: 577–593. [Google Scholar]
- Hansen M. 1996. Coleoptera Hydrophiloidea and Hydraenidae, Water Scavenger Beetles. In: Nilsson A. (Ed.), The Aquatic insects of North Europe. Stenstrup: Apollo Books, pp. 173–175. [Google Scholar]
- Hebauer F. 1994a. Entwurf einer Entomosoziologie aquatischer Coleoptera in Mitteleuropa (Insecta, Coleoptera, Hydradephaga, Hydrophiloidea, Dryopoidea). Lauterbornia 19: 43–57. [Google Scholar]
- Hebauer F. 1994b. Katalog der bayerischen Wasserkäfer, ihrer Ökologie, Verbreitung, Gefährdung. Ber Akad Natsch Landschaftspfl. 18: 47–59. [Google Scholar]
- Hurlbert SH. 1984. Pseudoreplication and the Design of Ecological Field Experiments. Ecol Monogr 54: 187–211. [CrossRef] [Google Scholar]
- Ivković M, Mičetić Stanković V, Mihaljević Z. 2012. Emergence patterns and microhabitat preference of aquatic dance flies (Empididae; Clinocerinae and Hemerodromiinae) on a longitudinal gradient of barrage lake system. Limnologica 42: 43–49. [CrossRef] [Google Scholar]
- Ivković M, Kesić M, Mihaljević Z, Kudela M. 2014. Emergence patterns and ecological associations of some haematophagous blackfly species along an oligotrophic hydrosystem. Med Vet Entomol 28: 94–102. [CrossRef] [Google Scholar]
- Ivković M, Miliša M, Baranov V, Mihaljević Z. 2015. Environmental drivers of biotic traits and phenology patterns of Diptera assemblages in karst springs: The role of canopy uncovered. Limnologica 54: 44–57. [CrossRef] [Google Scholar]
- Ivković M, Miliša M, Mihaljević Z. 2010. The aquatic dance flies fauna (Diptera, Empididae: Hemerodromiinae and Clinocerinae) of the Plitvice Lakes National Park. Nat Croat 19: 133–139. [Google Scholar]
- Jäch MA. 1982. Beitrag zur Kenntnis der Wasserkäfer des Bezirkes Scheibbs (NÖ) (Col. Elmidae, Hydraenidae excl. Limnebius, Dytiscidae). Koleopterol Rundsch 56: 75–88. [Google Scholar]
- Jäch MA. 1998. Annotated check list of aquatic and riparian/littoral beetle families of the world. In: Jäch MA, Ji L. (Eds.), Water beetles of China, Zoologisch-Botanische Gesellschaft in Österreich & Wiener Coleopterologenverein, pp. 25–42. [Google Scholar]
- Jäch MA, Balke M. 2008. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 595: 419–442. [Google Scholar]
- Jäch MA, Brojer M. 2006. Wasserkäfer. Skriptum zum Spezialpraktikum. Forschungsinstitut für Insektenkunde, Naturhistorisches Museum Wien, 426 pp. [Google Scholar]
- Jäch MA, Dietrich F, Raunig B. 2005. Rote Liste der Zwergwasserkäfer (Hydraenidae) und Krallenkäfer (Elmidae) Österreichs (Insecta: Coleoptera). In: Zulka KP (Ed.), Rote Listen gefährdeter Tiere Österreichs Checklisten, Gefährdungsanalyse, Handlungsbedarf. Part 1: Säugetiere, Vögel, Heuschrecken, Wasserkäfer, Netzflügler, Schnabelfliegen, Tagfalter. Grüne Reihe des Lebensministeriums, Vol. 14/1., Wien: Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wirtschaft, 211–284. [Google Scholar]
- Jäch MA, Kodada J, Brojer M, Shepard WD, Čiampor F. 2016. Coleoptera: Elmidae and Protelmidae. World Catalogue of Insects, Vol. 14. Leiden: Brill, 318 pp. [Google Scholar]
- Jäch MA, Skale A. 2015. Hydraenidae. In: Löbl I, Löbl D. (Eds.), Catalogue of Palaearctic Coleoptera, Vol. 2. Hydrophiloidea − Staphylinoidea, Revised and updated edition. Leiden: Brill, pp. 130–162. [Google Scholar]
- Janssens EL. 1965. Les Hydraena de l'Égéide − Mémoires. Académie royale de Belgique. Classe des Sciences. Deuxième série 16: 1–126. [Google Scholar]
- Klausnitzer B. 1996. Käfer im und am Wasser Bd. 567. Magdeburg: Westarp Wissenschaften. Heidelberg: Spektrum Akademischer Verlag (Die Neue Brehm-Bücherei), 127 pp. [Google Scholar]
- Kučinić M, Malicky H. 2002. Rhyacophila dorsalis plitvicensis, a new subspecies (Trichoptera: Rhyacophilidae) from Croatia. – Proc. 10th Int. Symp. Trich., Nova Suppl. Ent. (Keltern) 15: 145–147. [Google Scholar]
- Maitland PS. 1967. The ecology of four species of Elminthidae in a Scottish river. Arch Hydrobiol 63: 104–122. [Google Scholar]
- Makjanić B. 1971/72. O klimi užeg područja Plitvičkih jezera. Hrvatski geografski glasnik 33–34: 5–24 [In Croatian]. [Google Scholar]
- Matoničkin I, Pavletić Z. 1967. Hidrologija protočnog sistema Plitvičkih jezera i njegove ekološko-biocenološke značajke. Carsus Iugoslaviae 5: 83–126 [In Croatian]. [Google Scholar]
- Matoničkin I, Pavletić Z, Tavčar V, Krkač N. 1971. Limnološka istraživanja reikotopa i fenomena protočne travertinizacije u Plitvičkim jezerima. Prirodoslovna istraživanja, Acta Biologica 7: 1–88 [In Croatian]. [Google Scholar]
- Mičetić Stanković V, Jäch MA, Kučinić M. 2015. Annotated checklist of Croatian riffle beetles (Coleoptera: Elmidae). Nat Croat 24: 93–109. [CrossRef] [Google Scholar]
- Mičetić Stanković V, Jäch MA, Kučinić M. 2018. Ecological traits of water beetles in a karstic river from the Eastern Mediterranean region. Limnologica 71: 75–88. [CrossRef] [Google Scholar]
- Moog O, Hartmann A. (Eds.), Fauna Aquatica Austriaca, 3. Edition 2017. Wien: Federal Ministry of Agriculture, Forestry, Environment and Water Management. http://www.ecoprof.at/index.php/faunaaquaticaaustriaca.html. [Google Scholar]
- Mužinić J, Filipović M. 2006. The Plitvice Lakes: World's Natural heritage. Croat Med J 47: 1–3. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [CrossRef] [PubMed] [Google Scholar]
- Nilsson A. 1996. Aquatic insects of North Europe. A taxonomic handbook. Vol. 1. Stenstrup: Apollo Books, pp. 115–222. [Google Scholar]
- Nilsson AN. 2003. Dytiscidae. In: Löbl I, Smetana A (Eds.), Catalogue of Palearctic Coleoptera, Vol. 2. Stenstrup: Apollo Books, 35–78. [Google Scholar]
- Nilsson AN. 2015. A world catalogue of the family Dytiscidae or the diving beetles (Coleoptera, Adephaga). Version 1.I 2015: 298 pp. Availabe at: http://www2.emg.umu.se/projects/biginst/andersn. [Google Scholar]
- Novak P. 1952. Kornjaši Jadranskog primorja (Coleoptera). Zagreb: JAZU, 521 pp. [In Croatian] [Google Scholar]
- Novak P. 1970. Rezultati istraživanja kornjaša našeg otočja. Zagreb: JAZU, 75 pp. [In Croatian] [Google Scholar]
- Olmi M. 1976. Fauna d'Italia. Vol. XII. Coleoptera: Dryopidae, Elminthidae. Vol. XII. Bologna: Edizioni Calderini, 280 pp. [Google Scholar]
- Pavletić Z. 1968. Flora mahovina Jugoslavije. Zagreb: Institut za botaniku, 431 pp. [In Croatian] [Google Scholar]
- Petrik M. 1958. Prinos hidrologiji Plitvica. In: Šafar J (Ed.), Nacionalni park “Plitvička jezera”. Zagreb: Poljoprivredni nakladni zavod, 49–172 [In Croatian]. [Google Scholar]
- Petrik M. 1961. Temperatura i kisik Plitvičkih jezera. Zagreb: JAZU, 37 pp. [In Croatian] [Google Scholar]
- Picazo F, Millán A, Dolédec S. 2012. Are patterns in the taxonomic, biological and ecological traits of water beetles congruent in Mediterranean ecosystems? Freshw Biol 48: 279–287. [Google Scholar]
- Pirisinu Q. 1981. Palpicorni (Coleoptera: Hydraenidae, Helophoridae, Spercheidae, Hydrochidae, Hydrophilidae, Sphaeridiidae). Perugia: Consiglio Nazionale delle Ricerche, 97 pp. [Google Scholar]
- Plenković-Moraj A, Horvatinčić N, Primc-Habdija B. 2002. Periphyton and its role in tufa deposition in karstic waters (Plitvice Lakes, Croatia). Biologia (Bratisl.) 57: 423–431. [Google Scholar]
- Poje D. 1989. Pregled klimatskih karakteristika Nacionalnog parka “Plitvička jezera”. Plitvički bilten 2: 87–99 [In Croatian]. [Google Scholar]
- Popijač A, Sivec I. 2009. Diversity and distribution of stoneflies in the area of Plitvice Lakes National Park and along the Mediterranean river Cetina (Croatia). Aquat Insects 31: 731–742. [CrossRef] [Google Scholar]
- PPNPJ. 1984. Prostorni plan Nacionalnog parka Plitvičkih jezera: idejno rješenje. In: Nacionalni park Plitvička jezera, Republički komitet za građevinarstvo, s.i.k.p.i.z.č.o., Zagreb and Republički zavod za zaštitu prirode, Zagreb i Plitvička jezera. [In Croatian] [Google Scholar]
- Previšić A, Dvorski P, Cetinić K, Ivković M. 2013. New records for the Croatian caddisfly (Trichoptera, Insecta) fauna from the Plitvice Lakes National Park. Entomol Croat 17: 7–12. [Google Scholar]
- Previšić A, Graf W, Kučinić M. 2010. Caddisfly (Trichoptera) fauna of the Plitvice Lakes National Park, Croatia. Denisia 29: 287–294. [Google Scholar]
- Ribera I. 2000. Biogeography and conservation of Iberian water beetles. Biol Conserv 92: 131–150. [CrossRef] [Google Scholar]
- Riđanović J. 1994. Geografski smještaj (položaj) i hidrogeografske značajke Plitvičkih jezera. In: Plitvička jezera-nacionalno dobro Hrvatske, svjetska baština. Zagreb: Uprava Nacionalnog parka Plitvička jezera, pp. 29–42 [In Croatian]. [Google Scholar]
- Sánchez-Fernández D, Abellán P, Mellado A, Velasco J, Millán A. 2006. Are water beetles good indicators of biodiversity in Mediterranean aquatic ecosystems? The case of the Segura river basin (SE Spain). Biol Conserv 15: 4507–4520. [Google Scholar]
- Sánchez-Fernández D, Bilton DT, Abellán P, Ribera I, Velasco J, Millán A. 2008. Are the endemic water beetles of the Iberian Peninsula and the Balearic Islands effectively protected? Biol Conserv 141: 1612–1627. [CrossRef] [Google Scholar]
- Schlosser JK. 1877. Fauna kornjašah Trojedne Kraljevine. Zagreb: JAZU, pp. 88–118 [In Croatian]. [Google Scholar]
- Smith AJE. 2004. The moss flora of Britain and Ireland. Cambridge: Cambridge University Press, 1012 pp. [Google Scholar]
- SPSS Inc., 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc. [Google Scholar]
- Srdoč D. 1985. Procesi taloženja kalcita u krškim vodama s posebnim osvrtom na Plitvička jezera. Carsus Iugoslaviae 11: 4–6 [In Croatian]. [Google Scholar]
- Ter Braak CJF, Šmilauer P. 2012. CANOCO reference manual and user's guide: software for ordination, version 5.0. Wageningen: Biometris. [Google Scholar]
- Verberk WCEP, Van Kleef HH, Dijkman M, Van Hoek P, Spierenburg P, Esselink H. 2005. Seasonal changes on two different spatial scales: response of aquatic invertebrates to water body and microhabitat. Insect Sci 12: 263–280. [CrossRef] [Google Scholar]
- Vigna Taglianti A, Audisio PA, Biondi M, et al. 1999. A proposal for a chorotype classification of the Near East fauna, in the framework of the Western Palearctic region. Biogeographia 20: 31–59. [Google Scholar]
- Vilenica M, Gattolliat JL, Ivković M, et al. 2014. The mayfly fauna (Insecta, Ephemeroptera) of the Plitvice Lakes National Park, Croatia. Nat Croat 23: 349–363. [Google Scholar]
- Zuur AF, Ieno EN, Smith GM. 2007. Analysing ecological data. New York: Springer Science, 672 pp. [Google Scholar]
Cite this article as: Mičetić Stanković V, Jäch MA, Ivković M, Stanković I, Kružić P, Kučinić M. 2019. Spatio-temporal distribution and species traits of water beetles along an oligotrophic hydrosystem: a case study. Ann. Limnol. - Int. J. Lim. 55: 22.
All Tables
Characteristics of the sampling sites in Plitvice Lakes National Park. Abbreviations: SBR: Spring of the Bijela rijeka, URBR: Upper reach of the Bijela rijeka, SCR: Spring of the Crna rijeka, MRCR: Middle reach of the Crna rijeka, LRCR: Lower reach of the Crna rijeka, TBLB: Tufa barrier Labudovac, TBKM: Tufa barrier Kozjak-Milanovac, TBNB: Tufa barrier Novakovića Brod, PS: Plitvica stream, RK: river Korana in the village of Korana; A: angiosperms, B: bryophytes, p: pebbles, s: stones, tc: tufa chunks.
Taxonomic list, abundance, species richness and distribution of larval and adult stages of water beetles in Plitvice Lakes NP from February 2007 to January 2008. Abbreviations: Lv.: larval stage, Ad.: adult stage; S2: microhabitat with angiosperms; S3: microhabitat with bryophytes. Sampling sites are abbreviated as in Table 1.
Seasonal dynamics of water beetles at 12 microhabitats from February 2007 to January 2008 in Plitvice Lakes NP.
All Figures
 |
Fig. 1 Position of Plitvice Lakes NP in Croatia. (b) Sampling sites in Plitvice Lakes NP. 1: Spring of the Bijela rijeka (SBR), 2: Upper reach of the Bijela rijeka (URBR), 3: Spring of the Crna rijeka (SCR), 4: Middle reach of the Crna rijeka (MRCR), 5: Lower reach of the Crna rijeka (LRCR), 6: Tufa barrier Labudovac (TBLB), 7: Tufa barrier Kozjak-Milanovac (TBKM), 8: Tufa barrier Novakovića Brod (TBNB), 9: River Korana in village of Korana (RK), 10: Plitvica stream (PS). *Sites from 6 to 8 are also presented in lateral profile because they are located at barriers between adjacent lakes. |
| In the text | |
 |
Fig. 2 Temporal variation in abundance of water beetles and overall adult/larvae ratio from February 2007 to January 2008 in Plitvice Lakes NP. |
| In the text | |
 |
Fig. 3 NMDS analysis of microhabitats in Plitvice Lakes NP from February 2007 to January 2008. Abbreviations: S2: microhabitat with angiosperms; S3: microhabitat with bryophytes. For locality abbreviations, see Figure 1. |
| In the text | |
 |
Fig. 4 Longitudinal distribution of water beetles in Plitvice Lakes NP. Abbreviations of biocoenotic regions: EUC: Eucrenal, HYC: Hypocrenal, ER: Epirhithral, MR: Metarhithral, HR: Hyporhithral, EP: Epipotamal, MP: Metapotamal, LT: Littoral. For locality abbreviations, see Figure 1. |
| In the text | |
 |
Fig. 5 Biogeography of identified water beetles in Plitvice Lakes NP. Abbreviations of biogeographical chorotypes: AE: Asiatic; B: Balkan; BT: Balkano-Turanian; CAE: Centralasiatic-European; E: European; TE: Turano-European; TEM: Turano-Europeo-Mediterranean. |
| In the text | |
 |
Fig. 6 Seasonal dynamics and adult/larvae ratio of Elmis bosnica in the Bijela rijeka and the Crna rijeka from February 2007 to January 2008 at: a) springs and b) along the watercourse. Abbreviations: Ad.: adult stage, Lv.: larval stage; S2: microhabitat with angiosperms; S3: microhabitat with bryophytes. For locality abbreviations, see Figure 1. |
| In the text | |
 |
Fig. 7 Canonical correspondence analysis of water beetles in Plitvice Lakes NP. Abbreviations: Elm_a_r: Elmis aenea/rioloides, Lim_vol_sp: Limnius volckmari/sp., Riol_cup_sub: Riolus cupreus/subviolaceus. Other taxa are abbreviated as in Table 2. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


