| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 54, 2018
|
|
|---|---|---|
| Article Number | 28 | |
| Number of page(s) | 7 | |
| DOI | https://doi.org/10.1051/limn/2018020 | |
| Published online | 22 August 2018 | |
Research Article
Combined effects of oxytetracycline concentration and algal food level on the life-table demography of Brachionus calyciflorus (Rotifera)
Provincial Key Laboratory of Conservation and Utilization of important Biotic Resources in Anhui Province, Center of Cooperative Innovation for Recovery and Reconstruction of Degraded Ecosystem in Wanjiang City Belt, College of Life Sciences, Anhui Normal University,
Wuhu
241000, PR China
* Corresponding author: ylxi1965@126.com
Received:
29
March
2018
Accepted:
25
June
2018
Oxytetracycline (OTC) is commonly used for aquaculture and livestock, and its environmental concentration has increased to a considerable level and displays potential environmental risk. In the present study, the life-table demography of Brachionus calyciflorus exposed to sublethal concentrations (30.0, 60.0, 90.0, 120.0, 150.0 and 180.0 mg L−1) of OTC was investigated at 1.0 × 106, 2.0 × 106 and 4.0 × 106 cells mL−1 of Scenedesmus obliquus. The results showed that at each algal density, OTC concentration affected significantly life expectancy at hatching, net reproductive rate, generation time and intrinsic rate of population increase (P < 0.01), but did not affect proportion of mictic offspring of the rotifers (P > 0.05). Compared to the controls, and at 2.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 30.0–150.0 and 60.0–120.0 mg L−1 significantly prolonged life expectancy at hatching and generation time, treatments with OTC at 30.0–120.0 and 30.0–90.0 mg L−1 increased net reproduction rate and intrinsic rate of population increase, respectively, but the reverse was also true for those with OTC at 180.0 mg L−1. Higher and lower algal densities decreased the magnitude of stimulatory effects of lower concentrations of OTC but enhanced that of inhibitory effects of high concentration of OTC on the survival, asexual reproduction and population growth of the rotifers. At the three algal densities, net reproduction rate was more sensitive to OTC than the other endpoints, and significant concentration- effect relationship existed between OTC concentration and each of all the life-table demographic parameters except the proportion of mictic offspring.
Key words: Rotifer / antibiotics / algal density / chronic toxicity / life history
© EDP Sciences, 2018
1 Introduction
Antibiotics are pharmaceuticals widely used not only for human and veterinary medication but also for livestock and aquaculture growth promotion (Sarmah et al., 2006). After normal application, 50–90% of antibiotics and/or their metabolites are excreted from the body via feces or urine and enter the environment indirectly or directly (Schlusener and Bester, 2006). Contamination of water and soil systems with low concentrations of antibiotics may create bacteria resistant to antibiotics and then public health problems (Mulamattathil et al., 2014; Poonia et al., 2014). In recent years, the occurrence of antibiotics in aquatic ecosystems and their effects have received increasing attention by the scientific community (Sarmah et al., 2006; Kümmerer, 2009; Aarestrup, 2012).
Oxytetracycline (OTC), originally isolated from Streptomyces in soil, is a broad-spectrum antibiotic against gram-negative and positive bacteria. Due to its low cost, high efficiency and practicability, OTC was reported as pharmaceutical of high usage and high potential risk for the aquatic environment in England, Korea and China (Jones et al., 2002; Lee et al., 2008; Ji et al., 2012), and showed acute and chronic toxicity to organisms of different trophic levels, such as bacteria, algae, invertebrates and fish (Carvalho and Santos, 2016).
Zooplankton is frequently used to detect anthropogenic contamination because of their sensitivity to various toxicants and their important role in aquatic ecosystems. Among them, rotifers, especially Brachionus calyciflorus and Brachionus plicatilis, are frequently used in aquatic ecotoxicological studies (Snell and Janssen, 1995; Dahms et al., 2011). With rotifers as test animals, Isidori et al. (2005) investigated the LC50 and EC50 values of OTC to B. calyciflorus, Göksan and Gökpinar (2001) studied the effects of OTC on the activity and mortality of B. plicatilis, and Rotman (2011) assessed the effects of OTC on population growth of B. plicatilis, but they did not deal with the effects on life-table demographic parameters which are the important theoretical bases to measure the effects of chronic exposure of populations to toxicants (Ferrando et al., 1996).
Monogonont rotifers, such as B. calyciflorus and B. plicatilis, have a cyclical parthenogenesis mode of reproduction. Parthenogenesis dominates the monogonont life cycle where reproduction occurs in the absence of males (amicitc phase). After a period of clonal propagation, a sexual phase starts, induced by environmental factors such as population density, when some asexual females start to parthenogenetically produce sexual daughters that produce haploid eggs through meiosis which develop either into haploid males or, if fertilized, into resting eggs (Wallace et al., 2006).
Food level is a key factor affecting not only the survival, reproduction and population growth of rotifers but also the toxicity of pollutants, and higher and lower algal densities enhance the negative effects of pollutants on the survival, reproduction and population growth of rotifers (Nandini and Sarma, 2000; Sarma et al., 2001; Pickhardt et al., 2002; Ramírez-Pérez et al., 2004; Pan et al., 2016). In the present study, we investigated the chronic toxicity of sublethal concentrations of OTC to Brachionus calyciflorus in relation to Scenedesmus obliquus level, and compared the relative sensitivity of various endpoints to OTC exposure, with the aim of testing the following two hypotheses: (i) lower concentrations of OTC had stimulatory effects on the survival, asexual reproduction and population growth of B. calyciflorus, (ii) the sensitivity of life-table demographic parameters of B. calyciflorus to OTC varied with algal density, based on the effects of rifampicin on the survival, asexual reproduction and population growth of B. calyciflorus (Zhai et al., 2016).
2 Materials and methods
2.1 Collection and culture of test animals
An individual of B. calyciflorus was obtained by hatching a resting egg collected from sediments of Lake Fengming (31°20′N, 119°21′E), identified morphologically under a microscope based on the taxonomic description by Koste (1978), and then clonally cultured under controlled laboratory conditions. Stock rotifer culture had been kept under static-renewal conditions with a 16: 8 h light: dark photoperiod at 130 lx at 25 ± 1 °C in an illumination incubator for over 3 months, with EPA medium (96 mg NaHCO3, 60 mg CaSO4 · 2H20, 60 mg MgSO4 and 4 mg KCl per liter of distilled water; USEPA, 1993) as culture medium and the green algae S. obliquus as food. Before the experiments commenced, rotifers were cultured at 1.0 × 106, 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus for at least 2 weeks. During acclimation, every 24 h residual food and resting eggs, if produced, in the bottom of each glass tube were eliminated, the culture medium was renewed, and the chosen density of S. obliquus was supplied. Algae were grown in a semi-continuous culture using HB-4 medium (Li et al., 1959) renewed daily at 20%. Algae in exponential growth were centrifuged and resuspended in EPA medium.
2.2 Preparation of test solutions
Oxytetracycline hydrochloride (99.0% purity) used in the present study was purchased from Beijing Solarbio Technology Co. Ltd., China. Stock solution of 900 mg L−1 was carefully prepared by dilution of OTC in distilled water, and kept refrigerated and protected from light. Test solutions were prepared immediately before the beginning of the test by successive dilution of the stock.
2.3 Life table experiments
Based on the LC50-value (34.21 mg L−1) of Isidori et al. (2005), we selected seven toxicant concentrations (0 (control), 30.0, 60.0, 90.0, 120.0, 150.0 and 180.0 mg L−1) for the life-table experiments. Considering the algal densities in eutrophic water bodies (Pickhardt et al., 2002), we chose three densities (1.0 × 106, 2.0 × 106 and 4.0 × 106 cells mL−1) of S. obliquus. For each toxicant concentration and food level combination, four replicates were set up. Life-table experiments were conducted in 8 mL glass cups each containing 5 mL test solution with the chosen density of algal food. We started the experiments by introducing 10 neonates (<4 h old) into each of the 84 test cups (seven toxicant concentrations × three food levels × four replicates). The experiments were conducted in darkness at 25±1 °C. During the experiments, every 12 h the number of initial individuals and the number of produced neonates (amictic and mictic) were counted under a microscope. Dead individuals were removed, and the neonates were moved into another fresh cup and cultured at the same conditions as stated above until they produced their eggs. Based on the egg type (amictic or mictic eggs), the female type of neonate (amictic or mictic female) was identified and the proportion of mictic offspring was calculated as the number of mictic offspring divided by the number of amictic and mictic offspring. Every 24 h the initial rotifers alive were transferred into freshly prepared test solution. The experiments were terminated when all initial individuals died (Birch, 1948).
Based on the data collected, age-specific survival (lx) and age-specific fecundity (mx) were constructed for each cohort using conventional life-table techniques (Poole, 1974), and life expectancy at hatching (e0), generation time (T), net reproductive rate (R0) and intrinsic rate of population increase (rm) were calculated according to Krebs (1985) and Lotka (1913):
Net reproductive rate: 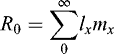
Generation time: 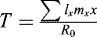
Intrinsic rate of population increase (r), first an approximation using: r-rough = lnR0/T
For final calculation, we solved the equation: 
2.4 Statistical analyses
All statistical analyses were performed using SPSS 11.5. The Levene's test was performed to test the homogeneity of variances. Kaplan–Meier analyses were conducted to test for the differences in the age-specific survivorships of the rotifer cohorts among the seven groups at each food level. One-way analysis of variance (ANOVA) was conducted to identify the significant effect of OTC concentration on each of the life history variables of the rotifers cultured at each algal density, and two-way ANOVA was conducted to analyze the significant effects of toxicant concentration, food level and their interactions on each life-history variable. Multiple comparisons of the least significant difference were performed to determine which groups were significantly different among the seven groups at each food level. The relationships between OTC concentration and each of life-table demographic parameters were regressively analyzed. Results with P values of less than 0.05 were considered statistically significant.
3 Results
The age-specific survivorship and fecundity of B. calyciflorus exposed to different concentrations of OTC and cultured at three food densities are presented in Figure 1. Kaplan–Meier analyses revealed that OTC concentration significantly affected the survivorship of B. calyciflorus cultured at each algal density (P < 0.05). Compared with the controls, and at 1.0 × 106, 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, the rotifers exposed to OTC at 60.0–120.0, 30.0–150.0, and 90.0 and 120.0 mg L−1 survived longer, but the reverse was also true for those exposed to OTC at 150.0 and 180.0, 180.0, and 150.0 and 180.0 mg L−1, respectively (P < 0.05). The OTC concentration at which the rotifers had a maximum fecundity increased from 30.0 to 30.0 and 60.0, and 60.0 and 90.0 mg L−1 when the algal level increased from 1.0 × 106 to 2.0 × 106 and 4.0 × 106 cells mL−1 (Fig. 1).
Figure 2 shows the selected life history variables of B. calyciflorus subjected to different concentrations of OTC and cultured at three food densities. OTC concentration significantly affected all the selected life-table demographic parameters (P < 0.01) except the proportion of mictic offspring (P > 0.05) of B. calyciflorus cultured at each algal density. Compared with the control, and at 2.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 30.0–150.0 mg L−1 significantly prolonged the life expectancy at hatching. However, at both 1.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, only the treatment with OTC at 90.0 mg L−1 significantly prolonged it. At the three food levels, treatment with OTC at 180.0 mg L−1 shortened the life expectancy at hatching (Fig. 2).
Compared with the control, and at 2.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 30.0–120.0 mg L−1 significantly increased the net reproduction rate, and that with OTC at 180.0 mg L−1 decreased it. However, at 1.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 30.0 and 90.0 mg L−1 significantly increased the net reproduction rate, and those with OTC at 150.0 and 180.0 mg L−1 decreased it. At 4.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 30.0–90.0 mg L−1 significantly increased the net reproduction rate, and that with OTC at 180.0 mg L−1 decreased it (Fig. 2).
Compared with the control, and at 2.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 60.0–120.0 mg L−1 significantly prolonged the generation time. However, at 1.0 × 106 cells mL−1 of S. obliquus, no treatment significantly prolonged the generation time. At 4.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 90.0 and 120.0 mg L−1 significantly prolonged the generation time. At the three food levels, treatment with OTC at 180.0 mg L−1 shortened the generation time (Fig. 2).
Compared to the control, and at 2.0 × 106 cells mL−1 of S. obliquus, treatments with OTC at 30.0–90.0 mg L−1 significantly increased the intrinsic rates of population increase. However, at 1.0 × 106 cells mL−1 of S. obliquus, no treatment significantly increased the intrinsic rate of population increase. At 4.0 × 106 cells mL−1 of S. obliquus, treatment with OTC at 30.0 mg L−1 significantly increased the intrinsic rate of population increase. At the three food levels, treatment with OTC at 180.0 mg L−1 decreased the intrinsic rate of population increase (Fig. 2).
Two-way ANOVA showed that food level had significant effects on the life expectancy at hatching, the net reproduction rate and the intrinsic rate of population increase (P < 0.01), OTC concentration had marked effects on the life expectancy at hatching, the net reproduction rate, the generation time and the intrinsic rate of population increase (P < 0.01), and the interaction between food level and OTC concentration had a significant effect on the net reproduction rate (P < 0.01) (Tab. 1).
A clear concentration-effect relationship existed between OTC concentration and life expectancy at hatching, net reproductive rate, generation time as well as intrinsic rate of population increase (Tab. 2).
 |
Fig. 1 Age-specific survivorship (unfilled square) and fecundity (filled square) of B. calyciflorus exposed to different concentrations of OTC and cultured at three food densities (mean ± standard error). |
 |
Fig. 2 Life expectancy at hatching (e0), net reproduction rate (R0), generation time (T), intrinsic rate of population increase (rm) and proportion of sexual offspring (PS) of B. calyciflorus exposed to different concentrations of OTC and cultured at 1.0 × 106 (white bars), 2.0 × 106 (grey bars) and 4.0 × 106 (black bars) cells mL−1 of Scenedesmus obliquus (mean ± standard error). *Significant (P < 0.05) or **highly significant (P < 0.01) difference from the control at the same algal density. |
Effects of algal density and OTC concentration on life expectancy at hatching (e0), net reproduction rate (R0), generation time (T), intrinsic rate of population increase (rm) and proportion of sexual offspring (PS) of B. calyciflorous (Two-way ANOVA).
Relationships between life expectancy at hatching (e0, h), net reproduction rate (R0, ind female−1 life−1), generation time (T, h) and intrinsic rate of population increase (rm, d−1) of B. calyciflorus cultured at three algal densities and OTC concentration (x, mg L−1)
4 Discussion
Under the chronic stress of antibiotics including streptomycin sulfate, tetracycline hydrochloride, tylosin tartrate and amoxicillin, the average lifespan and/or the life expectancy at hatching of B. calyciflorus, B. plicatilis and B. havanaensis significantly shortened (Araujo and McNair, 2007; González-Pérez et al., 2016). However, lower concentrations of rifampicin markedly prolonged the average lifespan and the life expectancy at hatching of B. calyciflorus (Zhai et al., 2016). Similarly, in the present study, OTC at 90.0, 30.0–150.0 and 90.0 mg L−1 significantly prolonged the life expectancy at hatching of B. calyciflorus cultured at 1.0 × 106, 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, respectively. In addition, OTC at 180.0 mg L−1 shortened the life expectancy at hatching. The above stated results indicated that lower concentrations of rifampicin and OTC might have a stimulating effect on the survival of the rotifers, but higher concentrations of them might have a toxic effect.
As a consequence of chronic stress of streptomycin sulfate, tetracycline hydrochloride, tylosin tartrate and amoxicillin, a reduction in net reproduction rate was observed in B. calyciflorus, B. plicatilis and B. havanaensis (Araujo and McNair, 2007; González-Pérez et al., 2016). However, lower concentrations of rifampicin increased the net reproduction rate of B. calyciflorus cultured at 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus (Zhai et al., 2016). In the present study, OTC at 30.0 and 90.0, 30.0–120.0, and 30.0–90.0 mg L−1 significantly increased the net reproduction rate, but OTC at 150.0 and 180.0, 180.0 and 180.0 mg L−1 decreased the net reproduction rate of the rotifers cultured at 1.0 × 106, 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, respectively. It is worthy of further researching that how lower concentrations of OTC stimulate the reproduction of the rotifers.
Amoxicillin at 50–200 and 200 μg L−1 significantly shortened the generation time of B. calyciflorus and B. havanaensis, respectively (González-Pérez et al., 2016). However, rifampicin at 2.0–10.0 and 10.0 mg L−1 significantly prolonged the generation time of B. calyciflorus cultured at 1.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, respectively (Zhai et al., 2016). Similarly, in the present study, OTC at 60.0–120.0 and 90.0–120.0 mg L−1 significantly prolonged the generation time of the rotifers when they were cultured at 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, respectively. In addition, the present study also showed that OTC at 180.0 markedly shortened the generation time. The generation time is the average length of time between the birth of an individual and the birth of its own offspring. As such, it reflects changes in the time required to reach sexual maturity and the embryonic developmental time. The effects of sublethal concentrations of OTC on the time required to reach sexual maturity and the embryonic developmental time of the rotifers need investigation.
The results available now have showed that the effect of antibiotics on the intrinsic rate of population increase of rotifers varied with antibiotic species and concentration, rotifer species, and algal density (Wang et al., 2008; Rotman, 2011; González-Pérez et al., 2016; Zhai et al., 2016). Similarly, the present study showed that at 1.0 × 106 cells mL−1 of S. obliquus, OTC at 30.0–150.0 mg L−1 did not significantly affect the intrinsic rate of population increase of B. calyciflorus. However, at 2.0 × 106 and 4.0 × 106 cells mL−1 of S. obliquus, OTC at 30.0-90.0, and 30.0 mg L−1 increased significantly the intrinsic rate of population increase, respectively. At the three food densities, OTC at 180.0 mg L−1 significantly decreased the intrinsic rate of population increase of the rotifers.
Lower concentrations of OTC prolonged significantly the life expectancy at hatching and the generation time, and increased the net reproduction rate and the intrinsic rate of population increase of B. calyciflorous, which supported the hypothesis that lower concentrations of OTC had stimulatory effects on the survival, asexual reproduction and population growth of B. calyciflorus.
The inhibitory effects of higher concentrations of streptomycin sulfate, tetracycline hydrochloride, tylosin tartrate and amoxicillin on the survival, reproduction and population growth of rotifers are usually attributed to their toxicity (Araujo and McNair, 2007; González-Pérez et al., 2016), and the stimulatory effects of lower concentrations of rifampicin were attributed to their inhibition of bacteria which might be harmful to rotifers (Zhai et al., 2016). The mechanisms under the effects of sublethal concentrations of OTC on the survival, reproduction and population growth of B. calyciflorus might be the same as those above stated antibiotics.
Mictic female production is a prerequisite for resting egg production, and is modulated by internal and external factors (Gilbert, 2004). Zhai et al. (2016) found that at the three algal levels, rifampicin at 2.0–10.0 mg L−1 significantly increased the proportion of mictic offspring of B. calyciflorus. Different from those results, the present study showed that at the three algal levels, OTC at 30.0–180.0 mg L−1 did not significantly affect the proportion of mictic offspring of B. calyciflorus.
The sensitivity of life-table demographic parameters of B. calyciflorous to antibiotics varied with not only antibiotic species but also algal density (Araujo and McNair, 2007; Zhai et al., 2016). Identical results were obtained in the present study. At 1.0 × 106 cells mL−1 of S. obliquus, the net reproduction rate was the most sensitive to OTC contamination. At 2.0 × 106 cells mL−1 of S. obliquus, the life expectancy at hatching, the net reproduction rate and the intrinsic rate of population increase had the same sensitivity. At 4.0 × 106 cells mL−1 of S. obliquus, both the net reproduction rate and the intrinsic rate of population increase had the same sensitivity. These results supported the hypothesis that the sensitivity of life-table demographic parameters of B. calyciflorous to OTC varied with algal density.
5 Conclusion
At 2.0 × 106 cells mL−1 of S. obliquus, lower concentrations of OTC prolonged significantly the life expectancy at hatching and the generation time, and increased the net reproduction rate and the intrinsic rate of population increase, but higher concentrations of OTC shortened or decreased them. Both increase and decrease in algal density decreased the stimulatory effect of lower concentrations of OTC but enhanced the inhibitory effect of higher concentrations of OTC (Fig. 2). At the three algal densities, the net reproduction rate was the most sensitive to OTC, and significant concentration-effect relationships existed between OTC concentration and life expectancy at hatching, net reproductive rate, generation time as well as intrinsic rate of population increase. In natural water bodies, OTC pollution will change the dynamics patterns of B. calyciflorous population, the community structures and the functioning of ecosystems. The effect magnitude will vary with algal biomass in water bodies.
Acknowledgements
This work was supported by the Natural Science Foundation of China (31470015) and the Foundation of Provincial Key Laboratory of Biotic Environment and Ecological Safety in Anhui Province. We thank the Shenzhen Nobel Science and Technology Service Co., Ltd. for language editing service.
References
- Aarestrup F. 2012. Sustainable farming: get pigs off antibiotics. Nature 486: 465–466. [CrossRef] [PubMed] [Google Scholar]
- Araujo A, McNair J. 2007. Individual and population level effects of antibiotics on the rotifers, Brachionus calyciflorus and B. plicatilis. Hydrobiologia 593: 185–189. [CrossRef] [Google Scholar]
- Birch LC. 1948. The intrinsic rate of natural increase of an insect population. J Anim Ecol 17: 15–26. [CrossRef] [Google Scholar]
- Carvalho TI, Santos L. 2016. Antibiotics in the aquatic environments: a review of the European scenario. Environ Int 94: 736–757. [CrossRef] [PubMed] [Google Scholar]
- Dahms HU, Hagiwara A, Lee JS. 2011. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol 101: 1–12. [CrossRef] [PubMed] [Google Scholar]
- Ferrando MD, Sancho E, Andreu-Moliner E. 1996. Chronic toxicity of fenitrothion to an algae (Nannochloris oculata), a rotifer (Brachionus calyciflorus), and the cladoceran (Daphnia magna). Ecotoxicol Environ Saf 35: 112–120. [CrossRef] [PubMed] [Google Scholar]
- Gilbert JJ. 2004. Population density, sexual reproduction and diapause in monogonont rotifers: new data for Brachionus and a review. J Limnol 63: 32–36. [CrossRef] [Google Scholar]
- Göksan T. Gökpinar Ş. 2001. The effects of oxytetracycline and flumequine on the rotifers (Brachionus plicatilis O.F. Müller). EU J Fish Aquat Sci 18: 367–373. [Google Scholar]
- González-Pérez BK, Sarma SSS, Nandini S. 2016. Effects of selected pharmaceuticals (ibuprofen and amoxicillin) on the demography of Brachionus calyciflorus and Brachionus havanaensis (Rotifera). Egypt J Aquat Res 42: 341–347. [CrossRef] [Google Scholar]
- Isidori M, Lavorgna M, Nardelli A, Pascarella L, Parrella A. 2005. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci Total Environ 346: 87–98. [CrossRef] [PubMed] [Google Scholar]
- Ji K, Kim S, Han S, et al. 2012. Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: are the current environmental concentrations safe? Ecotoxicology 21: 2031–2050. [CrossRef] [PubMed] [Google Scholar]
- Jones OAH, Voulvoulis N, Lester JN. 2002. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res 36: 5013–5022. [CrossRef] [PubMed] [Google Scholar]
- Koste W. 1978. Rotatoria. In: Die Radertier Mitteleuropas. Vol. 2, Berlin, Stuttgart (West Germany): Gebruder Borntraeger. [Google Scholar]
- Krebs CJ. 1985. Ecology: the experimental analysis of distribution and abundance. New York: Harper and Row. [Google Scholar]
- Kümmerer K. 2009. Antibiotics in the aquatic environment: a review − Part I. Chemosphere 75: 417–434. [Google Scholar]
- Lee YJ, Lee SE, Lee DS, Kim YH. 2008. Risk assessment of human antibiotics in Korean aquatic environment. Environ Toxicol Pharmacol 26: 216–221. [CrossRef] [PubMed] [Google Scholar]
- Li S, Zhu H, Xia Y, et al. 1959. The mass culture of unicellular green algae. Act Hydrobiol Sin 4: 462–472 (in Chinese). [Google Scholar]
- Lotka AJ. 1913. A natural population norm. J Wash Acad Sci 3: 241–248. [Google Scholar]
- Mulamattathil SG, Bezuidenhout C, Mbewe M, Ateba CN. 2014. Isolation of environmental bacteria from surface and drinking water in mafikeng, South Africa, and characterization using their antibiotic resistance profiles. J Pathog 2014: 371208. [CrossRef] [PubMed] [Google Scholar]
- Nandini S, Sarma SSS. 2000. Life table demography of four cladoceran species in relation to algal food (Chlorella vulgaris) density. Hydrobiologia 435: 117–126. [CrossRef] [Google Scholar]
- Pan L, Xi Y-L, Li Z-C, Zhao Q-Q, Hu Z-J. 2016. Effects of mercury on the life table demography of the rotifer Brachionus calyciflorus under different algal food (Scenedesmus obliquus) densities. Acta Ecol Sin 36: 218–223. [CrossRef] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. PNAS Retrieves 99: 4419–4423. [CrossRef] [Google Scholar]
- Poole RW. 1974. An introduction to quantitative ecology. New York: McGraw-Hill. [Google Scholar]
- Poonia S, Singh TS, Tsering DC. 2014. Antibiotic susceptibility profile of bacteria isolated from natural sources of water from rural areas of East sikkim. Indian J Community Med 39: 156–160. [CrossRef] [PubMed] [Google Scholar]
- Ramírez-Pérez T, Sarma SSS, Nandini S. 2004. Effect of mercury on the life table demography of the rotifer Brachionus calyciflorus Pallas (Rotifera). Ecotoxicology 13: 535–544. [CrossRef] [PubMed] [Google Scholar]
- Rotman FJ. 2011. Efficacy of a commercial probiotic relative to oxytetracycline as gram-negative bacterial control agents in a rotifer (Brachionus plicatilis) batch culture. North Am J Aquac 73: 343–349. [CrossRef] [Google Scholar]
- Sarma SSS, Nandini S, Ramírez-Pérez T. 2001. Combined effects of mercury and algal food density on the population dynamics of Brachionus patulus (Rotifera). Bull Environ Contam Toxicol 67: 841–847. [PubMed] [Google Scholar]
- Sarmah AK, Meyer MT, Boxall AB. 2006. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65: 725–759. [CrossRef] [PubMed] [Google Scholar]
- Schlusener MP, Bester K. 2006. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ Pollut 143: 565–571. [CrossRef] [Google Scholar]
- Snell TW, Janssen C. 1995. Rotifers in ecotoxicology: a review. Hydrobiologia 313/314: 231–247. [CrossRef] [Google Scholar]
- USEPA. 1993. Methods for measuring the acute toxicity of effluents to freshwater and marine organisms, 4th ed. Washington, DC: US Environment Protect Agency, EPA-600-4-90-027F. [Google Scholar]
- Wallace RL, Snell TW, Ricci C. 2006. Rotifera. Vol 1: biology, ecology and systematics. In Segers H, Dumont HJF, eds. Guides to the identification of the microinvertebrates of the continental waters of the World 23, Kenobi Productions, The Hague: Ghent/Backhuys Academic Publishing, pp. 69–70. [Google Scholar]
- Wang J-Q, Zhou Y-P, Lin J-J, Wang T-Y. 2008. Effects of two antibiotics on the population growth of rotifer Brachionus plicatilis. J Fudan Univ 47: 347–353 (in Chinese). [Google Scholar]
- Zhai P, Wen X-L, Chen Z-W, Zhao Z, Li H-Y, Xi Y-L. 2016. Effects of rifampicin on life table demography of Brachionus calyciflorus under different Scenedesmus obliquus densities. China Environ Sci 36: 1886–1894 (in Chinese). [Google Scholar]
Cite this article as: Jiang S, Xi Y-L, Zhu H, Zhang B-X, Yu J-H. 2018. Combined effects of oxytetracycline concentration and algal food level on the life-table demography of Brachionus calyciflorus (Rotifera). Ann. Limnol. - Int. J. Lim. 54: 28
All Tables
Effects of algal density and OTC concentration on life expectancy at hatching (e0), net reproduction rate (R0), generation time (T), intrinsic rate of population increase (rm) and proportion of sexual offspring (PS) of B. calyciflorous (Two-way ANOVA).
Relationships between life expectancy at hatching (e0, h), net reproduction rate (R0, ind female−1 life−1), generation time (T, h) and intrinsic rate of population increase (rm, d−1) of B. calyciflorus cultured at three algal densities and OTC concentration (x, mg L−1)
All Figures
 |
Fig. 1 Age-specific survivorship (unfilled square) and fecundity (filled square) of B. calyciflorus exposed to different concentrations of OTC and cultured at three food densities (mean ± standard error). |
| In the text | |
 |
Fig. 2 Life expectancy at hatching (e0), net reproduction rate (R0), generation time (T), intrinsic rate of population increase (rm) and proportion of sexual offspring (PS) of B. calyciflorus exposed to different concentrations of OTC and cultured at 1.0 × 106 (white bars), 2.0 × 106 (grey bars) and 4.0 × 106 (black bars) cells mL−1 of Scenedesmus obliquus (mean ± standard error). *Significant (P < 0.05) or **highly significant (P < 0.01) difference from the control at the same algal density. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


