| Issue |
Int. J. Lim.
Volume 58, 2022
|
|
|---|---|---|
| Article Number | 14 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/limn/2022015 | |
| Published online | 07 December 2022 | |
Research Article
The Effects of Environmental Variables on the Distribution of Immature Black Flies (Diptera, Simuliidae) in Various Streams of Northeastern Turkey
Hacettepe University, Faculty of Science, Department of Biology, 06800 Ankara, Turkey
* Corresponding author: ozzzge@gmail.com
Received:
7
March
2022
Accepted:
10
November
2022
The Eastern Black Sea Region of Turkey is located in the Caucasus Ecoregion and is one of the richest regions of the world in terms of biodiversity. Black flies are an important part of the biodiversity of running waters in the region. To determine the Simuliidae fauna of this region and understand the relationships between species distribution and environmental variables, 41 sites, mostly unpolluted or slightly polluted, were sampled in July 2008 and June 2009. A total of 3309 simulids and 20 species were identified. The most frequent and the most abundant species were Simulium variegatum Meigen, 1818, Simulium bezzii Corti, 1914, and Simulium trifasciatum Curtis, 1839. Simulium costatum Friedrichs, 1920, Simulium argenteostriatum Strobl, 1898, Simulium angustipes Edwards, 1915, Simulium balcanicum Enderlein, 1924, and Simulium pseudequinum Seguy, 1921 were positively correlated with NO2–N, water temperature, and pH but negatively correlated with PO4–P and dissolved oxygen. Simulium argyreatum Meigen, 1838, S. variegatum, and S. trifasciatum were positively correlated with dissolved oxygen. Simulium ornatum Meigen, 1818 was negatively correlated with pH. Prosimulium tomosvaryi Enderlein, 1921, Simulium cryophilum Rubtsov, 1959, Simulium vernum Macquart, 1826, and Simulium hispaniola Grenier and Bertrand, 1954 were negatively correlated with electrical conductivity. The results indicate that black flies, like other benthic macroinvertebrates, also respond to changes in environmental conditions with changes in composition and distribution. We hope that our research will contribute to biomonitoring studies in the future.
Key words: Canonical correspondence analysis / Eastern Black Sea Region / habitat preferences / bioindicator / physico-chemical variables
© EDP Sciences, 2022
1 Introduction
Aquatic insects dominate freshwater ecosystems and are important components of ecological dynamics (Hynes, 1970; Balian et al., 2008). As aquatic insects, black flies (Simuliidae) are a widespread family of the order Diptera. Larvae and pupae of this family are among the most common components of benthic macroinvertebrate communities in lotic systems (Cummins, 1987; Hamada et al., 2002). Larvae feed on organic particulates, filamentous algae, various insect larvae, zooplankton, phytoplankton, and bacteria in the water (Hershey et al., 1996; Ciborowski et al., 1997; Parkes et al., 2004; Wotton, 2009). As black flies can filter dissolved organic matter (DOM), they contribute to the food chain and play an important role in freshwater ecosystems (Zhang et al., 1998; Currie and Adler, 2008; Ciadamidaro et al., 2016).
Biomonitoring of the ecological quality of aquatic ecosystems is very important and necessary for the European Water Framework Directive (WFD) studies. Benthic macroinvertebrates are commonly used as bioindicators for monitoring habitat quality (Rosenberg and Resh, 1993; De Pauw et al., 2006). According to Hynes (1970), they are the best choice for evaluating river quality and are considered fingerprints of the river. Their abundance, composition, and diversity are important in determining the quality of habitat in aquatic ecosystems.
Black flies are also one of the important benthic macroinvertebrates taxa used in water quality assessment. Some species, that require specific environmental conditions, can be used as bioindicators for environmental quality and biological monitoring of freshwater habitats (Currie and Adler, 2008; Stangler et al., 2013; Lock et al., 2014). The aquatic stages of black flies are generally found in slightly polluted or unpolluted streams and rivers, however, some species are pollution-tolerant (Seitz, 1992; Feld et al., 2002). Many environmental factors affect the density, diversity, and distribution of black flies, including water temperature, dissolved oxygen concentration, substrate structure, riparian vegetation, current velocity, stream size, turbidity, pH, and electrical conductivity (Feld et al., 2002; Lautenschlager and Kiel, 2005; McCreadie et al., 2006; Landeiro et al., 2009; Rabha et al., 2013).
Simuliidae species have low tolerance to water temperature changes. This variable plays an important role in determining the species' composition due to the different temperature ranges preferred by the species (Rubtsov, 1990; Bernotiene and Bartkeviciene, 2013). Substrate structure is another environmental factor that affects the distribution of preimaginal black flies. They mostly prefer stones, submerged macrophytes, and leaves for clinging. A bottom structure with silt, sand, and gravel is not a suitable ground for the species. A change in current velocity causes this bottom material to move, causing individuals to drift (Rubtsov, 1990; Feld et al., 2002). Dissolved oxygen is also an important factor in blackfly community composition. Simuliidae species are generally found in well-oxygenated waters (Ciadamidaro et al., 2016; Lopez-Pena et al., 2020). The larvae and pupae of black flies need current to survive. Their density is usually high in streams and rivers with current velocity from moderate to high (Seitz, 1992; Zhang et al., 1998).
The Eastern Black Sea Region is particularly important for biodiversity and is a part of the Caucasus Biodiversity Hotspot (Myers et al., 2000; Kazancı et al., 2011). The Caucasus Ecoregion is one of the Global 200 Ecoregions described by the Worldwide Fund for Nature (WWF) and one of the world's 36 biodiversity hotspots (Hrdina and Romportl, 2017).
The objectives of this study were to (1) assess the taxonomic composition of Simuliidae in the stream ecosystems of the Eastern Black Sea Region, (2) explain the relationships between the recorded species and environmental variables using Canonical Correspondence Analysis, and (3) provide information on the habitat preferences of species.
2 Materials and methods
2.1 Study area
The Eastern Black Sea Region is located in the northeast of Turkey. The region consists of a mountain range, many rivers, and seasonal streams flowing from the mountains to the Black Sea. The coast of the Eastern Black Sea receives the largest amount of precipitation throughout the year, with an average annual rainfall of 2200 mm (Sensoy et al., 2008). There are many slightly polluted or unpolluted mountain streams in the region. These streams provide favorable conditions for many species of black flies.
The study area includes Giresun, Gümüşhane, Trabzon, and Rize provinces. In addition, Amasya, Sivas, and Tokat provinces bordering the region were also included in this study. The streams and valleys where the studied sites are located are as follows: Aksu Stream, Çaykara Stream, Fırtına Stream, İkizdere Stream, Kelkit Stream, Yeşilırmak River, Altındere Valley, Ayder Valley, Anzer Valley, Ovit Valley, Kümbet Valley (Fig. 1). The zone information of sites is given in Table 3.
Due to the topographic structure of the region, scattered settlements and sparse agricultural lands were observed in the study area. Most of the studied sites were not affected by anthropogenic disturbances (agricultural, industrial, and domestic activities). Sites 6–23, 32, 37, 39–41 were located far from agricultural and urban areas and there were no anthropogenic activities or habitat degradation in these sites. There are settlements and agricultural areas around Sites 1–5, 24–31 and they have been affected by agricultural activities and urban pollution.
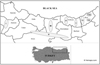 |
Fig. 1 Study area in Eastern Black Sea Region. (I: Yeşilırmak River (Sites 1, 2, 3), II: Kelkit Stream (Sites 24, 25, 26, 27, 28, 29, 30, 37, 38, 39, 40, 41), III: Kümbet Valley and Aksu Stream (Sites 4, 5, 31, 32, 33, 34, 35, 36), IV: Altındere Valley (Sites 6, 7), V: Çaykara Stream (Sites 8, 9), VI: Anzer Valley and İkizdere Stream (Sites 16, 17, 18, 19, 20, 21, 22, 23), VII: Ayder Valley and Fırtına Stream (Sites 13, 14, 15), VIII: Ovit Valley (Sites 10, 11, 12). |
2.2 Sampling and laboratory processing
Sampling was conducted in 41 sites in July 2008 and June 2009. Samples were taken from different parts of streams that reflect all the features of the sites using the semi-quantitative sampling method. One-time sampling was performed and the RIVPACS collecting protocol (Murray-Bligh, 1999; Wright, 2000) was applied in each site. Black fly samples were collected by three-minute kick-sampling using a standard D-shaped hand net (500 µm mesh size) with other benthic macroinvertebrates. These samples were washed through sieves with mesh sizes of 6 mm, 4 mm, 2 mm, and 500 µm. Additionally, larvae and pupae of blackflies attached on aquatic substrates such as stones, rocks, gravel, and submerged vegetation were collected by one-minute hand searching using fine forceps.
All the collected specimens were preserved in plastic bottles with 80% ethyl alcohol. In the laboratory, black fly specimens were separated from the rest of the benthic macroinvertebrates and they were identified to species level using a Leica MZ75 stereomicroscope and an Olympus CX21FS1 binocular microscope. Rubtsov (1990), Crosskey (2002), Jedlicka et al. (2004), Lechthaler and Car (2005), Crosskey and Zwick (2007) were used for identification.
In each site, water temperature, pH, electrical conductivity, and dissolved oxygen concentration were measured using the YSI 556 multi-probe system and current velocity was measured using a flow-meter, while benthic macroinvertebrate samples were collected. In addition, water samples were taken from 12 sites (1–5, 24–30) in July 2008 and 11 sites (31–41) in June 2009. They were stored at 4 °C until transported to the laboratory for the analyses of NO2–N, NO3–N, NH4–N, and PO4–P. Before analyses, each water sample was filtered using vacuum filtration to remove suspended particles. These analyses were measured in a HACH DR/890 data logging colorimeter, using a HACH kit according to the procedures of the handbook (HACH 2005). The water quality classes of the studied sites were evaluated using the Turkish Surface Water Quality Regulation (TSWQR, 2015). Klee (1991) was used to assess the water quality class according to PO4-P concentration (Tab. 1).
Water quality classes and ranges of the parameters.
2.3 Data analysis
The relationships between species of Simuliidae and environmental variables were investigated after log transformation through Canonical Correspondence Analysis (CCA) and absolute abundance was used in the analysis. CCA was performed in CANOCO 4.5 software package (ter Braak, 1986; ter Braak and Smilauer, 2002). Two separate CCA were conducted; the first (CCA1) was performed for 23 sites (Sites 1–5, 24–41), 15 species, and six physicochemical variables (water temperature, dissolved oxygen concentration, electrical conductivity, pH, NO2-N, and PO4-P) and the second (CCA2) was performed for 18 sites (Sites 6–23), 12 species, and four physicochemical variables (water temperature, dissolved oxygen concentration, pH, and electrical conductivity). The relative abundance and relative frequency of the species in each sampling site were also calculated.
In CCA diagrams, each quadrant was interpreted separately to indicate the relationship between physico-chemical variables and species. The physico-chemical variables were represented by arrows and the length of an arrow indicates the importance of this variable. The location of species relative to the arrows indicates the environmental preferences of each species. The species close to the arrow has a high correlation with the variable represented by the arrow.
3 Results and discussion
This is the first comprehensive study in the Eastern Black Sea Region. In this study, a total of 3309 black fly larvae and pupae belonging to 20 black flies species were collected from the 41 studied sites. These species were identified and characterized in terms of spatial distribution and physico-chemical variables. The number of black fly individuals at each site expresses the total of samples collected by hand and D-shaped net. The most frequently found species were S. variegatum, S. bezzii, and S. trifasciatum (41.5%, 34.1%, and 26.8% respectively) in this study. Likewise, these three species (S. bezzii, S. variegatum, and S. trifasciatum) were the most abundant species, representing 28.6%, 21.6%, and 11.1% of collected species, respectively (Tab. 2). Simulium variegatum was sampled from 17 sites and a total of 714 individuals. Simulium bezzii was sampled from 14 sites and a total of 948 individuals. Simulium trifasciatum was sampled from 11 sites and a total of 367 individuals. These species are widespread from Europe to the Caucasus (Adler, 2022).
The values of physicochemical variables (water temperature, pH, electrical conductivity, dissolved oxygen concentration, current velocity, NO2-N, NO3-N, NH4-N, and PO4-P) and the water quality classes of the sites are given in Table 3. Water temperature values ranged from 8.65 to 25.8 °C. pH values ranged from 4 to 8.72. Values of electrical conductivity ranged from 28 to 775 µS/cm. The concentrations of dissolved oxygen ranged from 3.11 to 12.18 mg/l. The current velocity values ranged from 0.38 to 2.33 m/s. The concentrations of ammonium ranged from 0 to 0.47 mg/l. The concentrations of nitrite ranged from 0 to 0.08 mg/l. The concentrations of nitrate ranged from 0 to 0.29 mg/l. The concentrations of orthophosphate ranged from 0 to 2.71 mg/l.
While the water quality classes of Sites 33-41 were Class III and Class IV according to PO4-P concentration and pH values, other physicochemical variables corresponded to Class I and Class II water quality. Although Sites 33-41 have good water quality, low pH values and high PO4-P concentrations were observed in these sites (Tab. 3). The values of these two variables may provide misleading information about the water quality of the sites. Therefore, the pH and PO4-P variables of these nine sites were ignored to determine the final water quality classes.
This event is known as episodic acidification, and it is a common event in the region. Kazancı (2009) reported this situation in the Eastern Black Sea Region and Yeşilırmak River Basin for the first time. Flooding caused by snowmelt and heavy rainfall in the spring and early summer seasons contributes to the short-term acidification of streams. The acid neutralization capacity has been reduced by transporting a large amount of organic acid into the stream. This causes a decrease in the pH value and an increase in the PO4-P concentration (Suzuki, 1982; Davies et al., 1993; Wellington and Driscoll, 2004).
Episodic acidification of the aquatic environment is a temporary situation, but it causes changes in the species composition and abundance of acid-sensitive aquatic organisms. Species of black flies are better able to cope with the short-term decline in the pH value than other aquatic macroinvertebrate groups (Bernard et al., 1990; Chmielewski and Hall, 1992). Therefore, some Simuliidae species (P. tomosvaryi, S. cryophilum, S. vernum) could be sampled in our studied sites, even under low pH conditions.
In this study, two separate CCA were applied to the data. Figure 2 shows the CCA1 diagram. The eigenvalues for axes 1–4 were 0.896, 0.598, 0.331, and 0.224 respectively in CCA1. All variables explain 38% of the total species variability. The species-environmental correlations for axis 1 (0.969) and axis 2 (0.862) were high, indicating a strong relationship between the environmental variables and black fly distribution. The total variance (inertia) in the species data was 5.999. The first two canonical axes accounted for 24.9% of the cumulative variance of the species data and 65.3% of the species-environment relationship. According to the Monte Carlo permutation test, indicating an association between environmental variables and black fly species, the first and all canonical axes were statistically significant (p ≤ 0.05). The order of significance from highest to lowest of the environmental variables in CCA1 was PO4-P, water temperature, dissolved oxygen concentration, pH, NO2-N, and electrical conductivity.
The CCA1 diagram includes 23 sites and 15 species recorded from these sites. The determinant environmental variables in quadrant A of the CCA1 diagram are NO2-N, water temperature, and pH. Sites 1–5, 24, 25, and 27 were placed in this quadrant. Sites 5 and 24 have Class II water quality, while other sites have Class III water quality. These sites in quadrant A have been affected by agricultural activities and urban pollution. The NO2-N concentrations in Sites 1, 2, 3, and 24 were high (0.03, 0.07, 0.08, and 0.04 mg/l, respectively) because of urban wastewater and agricultural runoff. Additionally, water levels in the riverbed of Sites 25 and 27 were low due to seasonal conditions, and habitat destruction was observed in these sites.
The species in this quadrant were S. costatum, S. argenteostriatum, S. angustipes, S. balcanicum, and S. pseudequinum. According to the CCA1 diagram, these species were positively correlated with NO2-N, water temperature, and pH but negatively correlated with PO4-P and dissolved oxygen. Simulium costatum lives generally in small, permanent, and undisturbed mountain streams (Rubtsov, 1990; Feld, 2005; Lechthaler and Car, 2005; Stangler et al., 2013). According to Schmedtje and Colling (1996), it prefers streams with moderate to high currents. Also, it is very resistant to low current velocity (Jensen, 1997). This species primarily prefers betamesosaprobic habitats, but it can also be found in oligosaprobic habitats (Lechthaler et al., 2017). The stream zonation preference of S. costatum is epirhithron mainly, but it also occurs in the hypocrenon and metarhithron zones of streams (Lechthaler et al., 2017). Simulium argenteostriatum mostly lives in unimpaired, fast-flowing mountain streams (Schmedtje and Colling, 1996; Lechthaler and Car, 2005; Stangler et al., 2013). It is generally found in oligosaprobic and betamesosaprobic habitats (Lechthaler et al., 2017). The stream zonation preferences of this species are epirhithron and metarhithron mainly, but it can also be found in the hyporhithron zone (Lechthaler et al., 2017). In this study, S. costatum and S. argenteostriatum were recorded only from Site 27, and one individual of each species was found in this site. Both species are moderately sensitive to changes in habitat quality and they prefer streams with high currents. Site 27, where habitat destruction has been observed and has low current velocity, does not have suitable habitat conditions for them. It is highly probable that these species, of which only one individual was collected, have drifted to Site 27 from the upper parts of the stream.
Simulium angustipes is an indicator species of eutrophic waters in lowland regions (Stangler and Halgos, 2007). It prefers small and warm waters in plain rivers (Lechthaler and Car, 2005; Ciadamidaro et al., 2016). This species is generally found in betamesosaprobic habitats, but it can also be found in oligosaprobic and alphamesosaprobic habitats (Lechthaler et al., 2017). The stream zonation preferences of this species are epipotamon and metapotamon mainly, but it also occurs in the metarhithron and hyporhithron zones of streams (Lechthaler et al., 2017). In this study, this species was recorded in Sites 25 and 27 with the highest water temperature values (25.41 and 25.79 °C, respectively). These two sites were located in the metarhithron zone of Kelkit Stream and have Class III water quality.
Simulium balcanicum inhabits mostly small-sized streams with slow-flowing (Rubtsov, 1990). Stangler and Halgos (2007) reported that it can tolerate high temperatures and survive in degraded aquatic habitats. This species mainly prefers betamesosaprobic habitats, but it can also be found in alphamesosaprobic habitats. The stream zonation preference of S. balcanicum is epipotamon mainly, but it also occurs in the hyporhithron and metapotamon zones of streams (Lechthaler et al., 2017). Simulium pseudequinum is found mostly in lowland streams and can tolerate habitat degradation and organic pollution (Lounaci et al., 2000). Many studies have reported that this species has a wide range of ecological preferences (Gallardo-Mayenco and Toja, 2002; Kazancı, 2006; Cherairia et al., 2014; Lopez-Pena et al., 2020). It mainly prefers betamesosaprobic habitats, but it can also be found in alphamesosaprobic habitats. The stream zonation preference of S. pseudequinum is epipotamon mainly, but it also occurs in the hyporhithron and metapotamon zones of streams (Lechthaler et al., 2017). In this study, S. balcanicum and S. pseudequinum were also recorded from the metarhithron zone of Kelkit Stream (Sites 24, 25, and 27). These three sites, and the other sites (Sites 1–5) where S. balcanicum and S. pseudequinum were recorded, have low current velocity and have Class II and Class III water quality because they have affected the agricultural areas and settlements around them. Domestic wastewater and agricultural activities increase the rate of nitrogen and organic matter input into aquatic environments (Helmer and Hespanhol, 1997; Lampert and Sommer 2007). Sites 2 and 3 also have the lowest dissolved oxygen concentrations (3.11 and 6.71 mg/l, respectively) and the high-temperature values (21.52 and 23.34 °C, respectively). It is an expected situation that they were placed opposite the arrow representing the dissolved oxygen variable.
There was no determinant environmental variable in quadrant B of the CCA1 diagram. Sites 31–36 were placed in this quadrant. Sites 31 and 32 have Class I water quality. In Sites 33, 34, 35, and 36, pH (5.5, 5.9, 4, and 5.5, respectively) and PO4-P values (2.02, 2.30, 2.02, and 1.88 mg/l, respectively) were associated with Class IV water quality because of episodic acidification, and other physicochemical variables were associated with Class I water quality.
The species in this quadrant were P. tomosvaryi, S. cryophilum, S. vernum, and S. hispaniola. Prosimulium tomosvaryi inhabits mostly mountain streams with fast-flowing and high dissolved oxygen concentrations (Scheder and Waringer 2002; Feld, 2005). In this study, the sites (Sites 32, 33, and 34) where this species was found, have high dissolved oxygen values (10.46, 9.04, and 9.02 mg/l, respectively) and have high current velocity. Lechthaler and Car (2005) reported that it can also be found in lowland streams with rich riparian vegetation. Simulium cryophilum is a widespread species of undisturbed mountain streams (Scheder and Waringer, 2002; Crosskey and Howard, 2004). Kazancı (2006) reported that this species can also be inhabited in cold and fast-flowing running waters. In the CCA1 diagram, it was recorded from Sites 32 and 33 with high water quality and fast-flowing. Simulium vernum can live in a wide range of stream types from spring to lowland rivers (Scheder and Waringer, 2002; Werner, 2008.). Simulium cryophilum and S. vernum which were found in Sites 32, 33, 35, and 36, were similarly recorded from four sites with episodic acidification in Camili (Artvin, Turkey) by Başören and Kazancı (2021). The common characteristic of the species in this quadrant is that all of them are mostly found in oligosaprobic and betamesosaprobic habitats, but they can also be inhabited in xenosaprobic and alphamesosaprobic habitats. Hypocrenon, epirhithron, metarhithron, and hyporhithron are stream zonation preferences of these species (Lechthaler et al., 2017). The stream zonation preferences of S. hispaniola are unknown. It was recorded at 2300 m from Ovit Mountain (Rize) and Yuvarlakçay Stream (Muğla) by Kazancı and Ertunç (2008). Lechthaler and Car (2005) and Ciadamidaro et al. (2016) reported that this species is generally found in cold mountain streams. In this study, a large number of S. hispaniola (120 individuals) were observed in Site 34. This site was located in the epirhithron zone of the Aksu Stream.
The species and sites in this quadrant were placed opposite the arrow representing electrical conductivity. Dissolved salts and nutrients entering the stream as a result of domestic, industrial, and agricultural pollution cause an increase in the electrical conductivity value (Morrison et al., 2001). Therefore, our results are consistent with the fact that Simuliidae species, which prefer clean waters and sites unaffected by pollution, were placed opposite the arrow representing the electrical conductivity variable.
The determinant environmental variable in quadrant C of the CCA1 diagram is electrical conductivity. Sites 26, 28, 29, 30, 38, and 40 were placed in this quadrant. Since Sites 28, 29, and 30 have high EC values (381, 364, and 341 µS/cm, respectively), they were positioned next to the arrow representing EC. Sites 26 and 30 have Class III water quality, Site 29 has Class II water quality, and Site 28 has Class I water quality. There are settlements and agricultural areas around Sites 28, 29, and 30, and these sites were located downstream from a dam. The water was released from the dam during sampling and measurement. Therefore, this situation caused changes in the physicochemical values of the water and some Simuliidae species may have drifted downstream. In Sites 28, 29, and 30, only two individuals of S. bezzii were found, but the recorded species and the water quality of these sites can be misleading. This species mostly lives in fast-flowing running waters, but it can also live in degraded eutrophic waters (Seitz, 1994; Lechthaler and Car, 2005). Sites 6, 10, 12, 21, 22, and 23 where the species was recorded, have high current velocity. It was also recorded in high numbers from eutrophic sites (172 individuals in Site 25 and 41 individuals in Site 27), which were located in quadrant A. Sites 38 and 40, which are the other sites in quadrant C were located in the epirhithron zone of Kelkit Stream. These sites have Class III and Class IV water quality due to the pH (6.4 in Site 38 and 6.37 in Site 40) and PO4–P values (2.23 mg/l in Site 38 and 2.71 mg/l in Site 40) caused by episodic acidification. But other physicochemical variables were associated with Class I and Class II water quality.
Another species in this quadrant was S. lineatum. According to Illesova et al. (2008), it is an indicator species of the eutrophic middle and lower sections of the Hron River (Slovakia). Feld (2005), also recorded this species from medium-sized lowland rivers. This species is generally found in betamesosaprobic habitats, but it can also be inhabited in alphamesosaprobic habitats. The stream zonation preference of S. lineatum is epipotamon mainly, but this species also occurs in the hyporhithron and metapotamon zones of streams (Lechthaler et al., 2017). In this study, it was recorded only from Site 26, and this site was located in the metarhithron zone of Kelkit Stream and has Class III water quality.
The determinant environmental variables in quadrant D of the CCA1 diagram are dissolved oxygen and PO4–P. Sites 37, 39, and 41 were placed in this quadrant. These three sites were located far from agricultural and urban areas. In these three sites, physicochemical variables (except PO4–P concentrations and pH values due to episodic acidification) correspond to the Class I and Class II water quality. This situation is temporary and periodic. During the rainy season, the pH values in streams decrease due to the snowmelt and the inflow of acidic rainwater (Vaananen et al., 2006; Kazancı, 2009). For this reason, Sites 37, 39, and 41 were placed opposite the arrow representing the pH variable. In addition, since these sites have high dissolved oxygen values (10.5 mg/l in Site 37, 8.94 mg/l in Site 39, and 12.18 mg/l in Site 41), they were located in the same quadrant with the dissolved oxygen variable. Site 41 was placed in the hypocrenon zone of the Kelkit Stream (Tomara Fall), and only Metacnephia subalpina Rubtsov, 1956 was recorded from this site. In this study, this species was not recorded in any other site. There is no information about the habitat preferences of M. subalpina in the literature. Crosskey and Zwick (2007) recorded this species from Pülümür Stream (Tunceli, Turkey), Sorgun Stream (Mersin, Turkey), and a stream near Ankara (Turkey). It was also recorded from the upper part of the Hrazdan River (Armenia) by Kachvoryan et al. (2007) and an unimpaired mountain stream (Artvin, Turkey) by Kazancı and Ertunç (2008).
Simulium ornatum, S. trifasciatum, and S. variegatum are other species in this quadrant. Simulium ornatum is one of the most common Simuliidae species and it can be found all over the Palearctic region (Adler, 2022). This species does not have special habitat requirements. In other words, it has a high tolerance to environmental variables (Zhang et al., 1998; Malmqvist et al., 1999; Feld et al., 2002; Ofenböck et al., 2002; Lautenschlager and Kiel, 2005; Lechthaler and Car, 2005). The most important environmental variable for the density of S. ornatum is pH. It is generally found in small, clean streams with a low pH (Bernotiene, 2006). In the CCA1 diagram, S. ornatum was placed opposite the arrow representing the pH variable. Its habitat preferences are mostly alphamesosaprobic and betamesosaprobic habitats, and it has a wide range of stream zonation preferences from the hypocrenon to metapotamon (Lechthaler et al., 2017). Simulium trifasciatum is positively associated with oxygen content and current velocity (Scheder and Waringer 2002). In the CCA1 diagram, it was placed relatively close to the arrow representing the dissolved oxygen variable. Stangler et al. (2013) reported that it is an indicator species in the undisturbed hyporhithral zone of streams. According to Lechthaler et al. (2017), it mainly prefers the epirhithron and metarhithron, but it also occurs in the hypocrenon and hyporhithron zones of streams, and it is generally found in oligosaprobic and betamesosaprobic habitats. Another species in this quadrant is Simulium variegatum. It is a typical inhabitant of mountain streams with fast currents and high dissolved oxygen concentrations (Feld et al., 2002; Kiel, 2001; Feld, 2005). Thusly, this species was placed near the arrow representing the dissolved oxygen variable. It is generally found in oligosaprobic and betamesosaprobic habitats, but it can also be inhabited in alphamesosaprobic habitats. The stream zonation preferences of S. variegatum are metarhithron and hiporhithron mainly, but it also occurs in the epirhithron zone of streams (Lechthaler et al., 2017). This species was also recorded from the crenon zone of streams (Sites 9, 17, and 22) in this study. Simulium trifasciatum and S. variegatum are two of the most frequent species (26.8 and 41.5 respectively) in this study, and their habitat preferences are compatible with the habitat structures of the sites (high dissolved oxygen concentrations, high current velocity) where they were recorded.
Figure 3 shows another CCA diagram (CCA2). The eigenvalues for axes 1–4 were 0.409, 0.208, 0.114, and 0.029 respectively in CCA2. All variables explain 22% of the total species variability. The species-environmental correlations for axis 1 (0.835) and axis 2 (0.834) were high, indicating a strong relationship between the environmental variables and black fly distribution. The total variance in the species data was 3.407. The first two canonical axes accounted for 18.1% of the cumulative variance of the species data and 81.1% of the species-environment relationship. According to the Monte Carlo permutation test, indicating an association between environmental variables and black fly species, the first and all canonical axes were statistically significant (p ≤ 0.05). The order of significance from highest to lowest of the environmental variables in CCA2 was electrical conductivity, dissolved oxygen concentration, water temperature, and pH.
The CCA2 diagram includes 18 sites and 12 species recorded from these sites. Simulium cryophilum, S. vernum, S. bezzii, S. hispaniola, S. ornatum, S. trifasciatum, and S. variegatum were common species in the CCA1 and CCA2 diagrams. The determinant environmental variable in quadrant A of the CCA2 diagram is water temperature. Only Site 6 was placed in this quadrant. Since this site has the highest water temperature value (18.22 °C) among the sites in the CCA2 diagram, it was located in the same quadrant with the arrow representing the temperature variable. Simulium bezzii, S. ornatum, and S. variegatum were recorded from Site 6 and two of them (S. bezzii and S. ornatum) were placed in quadrant A. Simulium ornatum was recorded only in Site 6 among all the sites in the CCA2 diagram. This species can be found in clean and polluted waters (Feld et al., 2002; Lechthaler and Car, 2005; Lautenschlager and Kiel, 2005). Environmental variables (except pH) are generally insignificant for the distribution of S. ornatum (Bernotiene, 2006). It is expected that this species, which prefers streams with low pH, was placed opposite the arrow representing the pH variable as in the CCA1 diagram. Simulium ornatum can live in a wide range of stream zones. Site 6 was located in the epirhithron zone of a stream in Altındere Valley (Trabzon). In this study, Simulium bezzii was the second most frequent species after S. variegatum. This species was recorded from 14 sites and has the highest number of individuals (559 individuals) in Site 6. Simulium bezzii can tolerate a wide range of water temperatures, dissolved oxygen concentrations, and water turbidity (Lopez-Pena and Jimenez-Peydro, 2020; Lopez-Pena et al., 2020). It was found primarily in the upper parts of streams with fast-flowing, but it can also survive in eutrophic waters with low dissolved oxygen concentrations (Lechthaler and Car, 2005; Chaoui Boudghane-Bendiouis et al., 2014). Kazancı and Ertunç (2010) reported for the first time that this species is resistant to morphological degradation of habitats. Simulium bezzii mainly prefers oligosaprobic and betamezosaprobic habitats (CSN 75 7716, 1998). There is no information about the stream zonation preferences of this species in the literature. It was recorded from the crenon, epirhithron, and metarhithron zones of the streams in this study.
The determinant environmental variable in quadrant B of the CCA2 diagram is electrical conductivity. Sites 9, 17, and 18 were placed in this quadrant and they have the highest electrical conductivity values (110, 110, and 87 µS/cm, respectively) among the sites in the CCA2 diagram. Site 9 has Class I water quality, Sites 17 and 18 have Class II water quality. Only S. variegatum was placed in quadrant B. This species is well adapted to high currents and is mostly found in cold streams with high dissolved oxygen concentrations (Feld et al., 2002; Kiel, 2001; Feld, 2005). Therefore, it was situated close to the arrow representing the dissolved oxygen variable in quadrant D. Simulium variegatum was also close to the center of the CCA2 diagram. This means that none of the environmental variables used in the CCA2 diagram influenced the occurrence of this species in the studied sites. Simulium variegatum is mostly found in oligosaprobic and betamesosaprobic habitats, but it can also be inhabited in alphamesosaprobic habitats. The stream zonation preferences of this species are metarhithron and hiporhithron mainly, but it also occurs in the epirhithron zone of streams (Lechthaler et al., 2017). It was also found in crenon zone of streams (Sites 9, 17, and 22) in this study. Lautenschlager and Kiel (2005) reported that it can live in degraded habitats. In this study, S. variegatum was the most frequent species, and it was mostly recorded from the upper parts of the streams with high oxygen content and high current velocity.
There was no determinant environmental variable in quadrant C of the CCA2 diagram. Sites 7, 10, 11, 19, 20, and 21 were placed in this quadrant. All of these sites have Class II water quality. They were located opposite the arrow representing the electrical conductivity variable because of low EC values (55 µS/cm in Site 7; 32 µS/cm in Site 10; 45 µS/cm in Site 11; 28 µS/cm in Site 19; 34 µS/cm in Sites 20 and 21). These electrical conductivity values indicate undisturbed habitats and these sites have not been influenced by any anthropogenic or agricultural activities.
The highest number of species (eight) was observed in this quadrant. These species were P. fulvipes, Prosimulium rufipes Meigen, 1830, Simulium angustitarse Lundström, 1911, S. cryophilum, S. vernum, S. hispaniola, S. trifasciatum, and Simulium auricoma Meigen, 1818. Prosimulium fulvipes inhabits mostly mountain streams with fast-flowing (Lechthaler and Car, 2005). It is most commonly found in xenosaprobic and oligosaprobic habitats (CSN 75 7716, 1998). There is no information about the stream zonation preferences of this species in the literature. It was recorded from the epirhithron zone of the streams (Sites 11, 19, and 20 with fast-flowing) in this study. Prosimulium rufipes mainly prefers oligosaprobic habitats, but it also occurs in xenosaprobic and betamesosaprobic habitats. The stream zonation preference of this species is epirhithron mainly, but it can also be found in the hypocrenon and metarhithron zones of streams (Lechthaler et al., 2017). Ofenböck et al. (2002) and Stangler et al. (2013) reported that this species is generally found in the undisturbed upper parts of streams with fast-flowing. Illesova et al. (2008) recorded P. rufipes from the upstream region of the Hron River Basin (Slovakia). In this study, it was recorded only from Site 20, which is located in the epirhithron zone of a stream with fast-flowing at 2500 m (Anzer Valley, Rize). Simulium angustitarse is a species widely distributed throughout Europe (Crosskey and Crosskey, 2002). It has a wide range of tolerance to ecological conditions (Lopez-Pena and Jimenez-Peydro, 2020). Car and Lechthaler (2002) recorded this species from a small lowland stream (in Bruck an der Leitha, Austria). It mainly prefers betamesosaprobic habitats, but it also occurs in oligosaprobic and alphamesosaprobic habitats. The stream zonation preferences of this species are epirhithron, metarhithron, and hyporhithron mainly, but it can also be found in the hypocrenon zone of streams (Lechthaler et al., 2017). In this study, it was recorded only from Site 21, which is located in the epirhithron zone of a stream at 2365 m (Anzer Valley, Rize). Simulium auricoma is a highly sensitive species to anthropogenic disturbances (Scheder, 2004). This species is usually found in mountain streams with cold and fast-flowing (Illesova and Halgos, 2004; Scheder, 2004; Talbalaghi et al., 2006). It is generally found in oligosaprobic habitats, but it can also be inhabited in xenosaprobic and betamesosaprobic habitats. Simulium auricoma prefers only the epirhithron zone of streams (Lechthaler et al., 2017). It was recorded from the epirhithron zone of streams with fast flowing in Site 10 (Ovit Valley, Rize) and Site 19 (Anzer Valley, Rize) in this study. The water temperatures of these sites (13.35 °C in Site 10 and 13.9 °C in Site 19) are suitable for the survival of S. auricoma. Simulium trifasciatum has a wide Palearctic distribution and it has a wide range of tolerance to ecological conditions (Lopez-Pena et al., 2020). Scheder and Waringer (2002) reported that it prefers upland streams with fast-flowing and high dissolved oxygen concentrations. Chaoui Boudghane-Bendiouis et al. (2014) recorded it from lowland sites with slow-flowing in the Tafna Basin (Algeria). This species is generally found in oligosaprobic and betamesosaprobic habitats. The stream zonation preferences of S. trifasciatum are epirhithron and metarhithron mainly, but it also occurs in the hypocrenon and hiporhithron zones of streams (Lechthaler et al., 2017). In this study, S. trifasciatum was the most frequent species, and it was mostly recorded from the upper parts of the streams with high oxygen content and high current velocity.
Simulium cryophilum, S. vernum, and S. hispaniola are the other species in quadrant C of the CCA2 diagram. These three species were also placed opposite the arrow representing the electrical conductivity variable in the CCA1 diagram. Simulium cryophilum inhabits mostly undisturbed mountain streams with fast-flowing (Scheder and Waringer, 2002; Kazancı, 2006; Crosskey and Howard, 2004). In the CCA2 diagram, it was recorded from Sites 18, 20, and 22 with high water quality and fast-flowing. Simulium vernum can be found in a variety of habitats, from small mountain streams to large rivers (Scheder and Waringer, 2002; Feld, 2005). They mainly prefer oligosaprobic and betamesosaprobic habitats, but they can also be inhabited in xenosaprobic and alphamesosaprobic habitats. Hypocrenon, epirhithron, metarhithron, and hyporhithron are stream zonation preferences of these three species (Lechthaler et al., 2017). There is no information about the habitat preferences of S. hispaniola in the literature. It was found in the epirhithron zone of the streams (Sites 7 and 34) in this study. This species is generally recorded in cold waters at high latitudes (Lechthaler and Car, 2005; Kazancı and Ertunç, 2008; Ciadamidaro et al., 2016). Habitat destruction and anthropogenic pollution were not observed at sites in this quadrant. The species in this quadrant (except S. angustitarse) are highly sensitive to organic pollution and hydromorphological degradation. Therefore, it is expected that these Simuliidae species and sites were placed together opposite the arrow representing the electrical conductivity variable associated with anthropogenic pollution.
The determinant environmental variables in quadrant D of the CCA2 diagram are pH and dissolved oxygen concentration. Sites 8, 12, 13, 14, 15, 16, 22, and 23 were placed in this quadrant. In these eight sites, physicochemical variables correspond to Class I and Class II water quality. There were no anthropogenic disturbances or habitat degradation in these sites. Only S. argyreatum was placed in quadrant D. This species is an indicator of undisturbed headwater streams and is sensitive to the morphological destruction of streams (Lautenschlager and Kiel, 2005). Stangler et al. (2013) recorded it from sub-mountainous rivers and large streams flowing through wide valleys (Carpathians, Slovakia). Ciadamidaro et al. (2016) recorded this species from medium-high courses of the streams and the Aniene River (Italy). According to Lechthaler et al. (2017), it mainly prefers epirhithron, metarhithron, and hyporhithron zones of streams and it is generally found in oligosaprobic and betamesosaprobic habitats. Simulium argyreatum was recorded in high numbers from Site 15 (72 individuals) and Site 22 (174 individuals). These sites were located in the metarhithron zone of the Fırtına Stream and the crenon zone of a stream in the Anzer Valley, respectively.
According to the CCA diagrams, S. costatum, S. argenteostriatum, S. angustipes, S. balcanicum, and S. pseudequinum were positively correlated with NO2–N, water temperature, and pH but negatively correlated with PO4–P and dissolved oxygen. Simulium. argyreatum, S. variegatum, and S. trifasciatum were positively correlated with dissolved oxygen. Simulium ornatum was negatively correlated with pH. Prosimulium tomosvaryi, S. cryophilum, S. vernum, and S. hispaniola were negatively correlated with electrical conductivity.
Distribution, relative abundance, and relative frequency of black fly species.
Environmental characteristics and water quality classes of the 41 studied sites. Abbreviations: SZ: Stream Zone, CV: Current Velocity, WT: Water Temperature, EC: Electrical Conductivity, DO: Dissolved Oxygen, FWQ: Final Water Quality Classes. *Sites with episodic acidification.
 |
Fig. 2 CCA1 diagram with selected environmental variables and Simuliidae species (•: Species, □: Sites). Abbreviations: P. tomosvaryi − P.tom; M. subalpina − M. sub; S. angustipes − S. ang; S. costatum − S. cos; S. cryophilum − S. cry; S. vernum − S. ver; S. argenteostriatum − S. arg; S. bezzi − S. bez; S. hispaniola − S. his; S. ornatum − S. orn; S. trifascitum − S. tri; S. variegatum − S. var; S. balcanicum − S. bal; S. lineatum − S. lin; S. pseudequinum − S. pse. |
 |
Fig. 3 CCA2 diagram with selected environmental variables and Simuliidae species (•: Species, □: Sites). Abbreviations: P. fulvipes − P. ful; P. rufipes − P. ruf; S. angustitarse − S. ang; S. cryophilum − S. cry; S. vernum − S. ver; S. argyreatum − S. arg; S. bezzi − S. bez; S. hispaniola − S. his; S. ornatum − S. orn; S. trifascitum − S. tri; S. variegatum − S. var; S. auricoma − S. aur. |
4 Conclusion
This research on the distribution and ecology of black flies in streams was conducted for the first time in the Eastern Black Sea Region. In this scope of the study, a total of 3309 black fly larvae and pupae belonging to 20 black fly species were recorded from 41 studied sites. The most frequent species were S. variegatum, S. bezzii, and S. trifasciatum in these sites. At the same time, these three species have the highest abundance in the study area. According to the Canonical Correspondence Analysis, water temperature, dissolved oxygen, pH, and electrical conductivity were important environmental parameters associated with black fly species distribution. The existence of different species of black flies could reflect the environmental conditions of freshwater ecosystems. The results of this study indicate that black fly species in streams in the research area serve as biological indicators in these lotic habitats.
In addition, this study also provides new information about the stream zonation preferences of some Simuliidae species (P. fulvipes, M. subalpina, S. bezzii, S. hispaniola, S. variegatum, S. balcanicum, S. lineatum, and S. pseudequinum). According to these results, P. fulvipes and S. hispaniola preferred the epirhithron zone, M. subalpina preferred the crenon zone, and S. bezzii preferred the crenon, epirhithron, and metarhithron zones of the streams in this study. In addition, S. balcanicum, S. lineatum, and S. pseudequinum which prefer the metapotamon, epipotamon, and hyporhithron zones of the streams were also found in the metarhithron zone of the streams in this study.
The aquatic fauna of streams is under threat due to increasing various anthropogenic activities (pollution, habitat alteration, and degradation) in the Eastern Black Sea Region. Some sensitive Simuliidae species may become extinct or decrease in species number, abundance, and diversity in the future. Therefore, ecological researches on black flies as well as other taxa are necessary for the protection of habitat quality. The information obtained from this study will provide important and unique contributions to the biomonitoring and biodiversity conservation studies to be carried out in the Eastern Black Sea Region. Furthermore, as the region is a biodiversity hotspot, extensive studies covering several seasons will allow more Simuliidae species to be recorded.
Acknowledgments
This research is a part of the Ph.D. thesis of Özge Başören. This work was financially supported by Hacettepe University Scientific Research Projects Coordination Unit (Project title: “A research on determination of Simuliidae (Insecta, Diptera) fauna of the Eastern Black Sea Region and habitat quality of species according to the European Union Water Framework Directive”, Project No: FHD-2015-7087). We would thank Hacettepe University for its financial support. We are grateful to G. Türkmen, P. Ekingen, Y. Gültutan, H. A. Bolat, and T. Tugaytimur for their help in the field and the laboratory.
References
- Adler P. 2022. World Blackflies (Diptera: Simuliidae): A comprehensive revision of the taxonomic and geographical inventory. 145 pp. [Google Scholar]
- Balian EV, Seger H, Martens K, Leveque C. 2008. The freshwater animal diversity assessment: an overview of the results. Hydrobiologia 595: 627–637. [CrossRef] [Google Scholar]
- Başören Ö, Kazancı N. 2021. Ecological requirements of larval Simuliidae (Insecta, Diptera) species of some streams in Camili Valley (Artvin, Turkey). Acta Aquat Turc 17: 97–107. [Google Scholar]
- Bernard DP, Neill WE, Rowe L. 1990. Impact of mild experimental acidification on short term invertebrate drift in a sensitive British Columbia stream. Hydrobiologia 203: 63–72. [CrossRef] [Google Scholar]
- Bernotiene R. 2006. On the distribution of black fly larvae in small lowland rivers in Lithuania. Acta Entomol Serb 11: 115–125. [Google Scholar]
- Bernotiene R, Bartkeviciene G. 2013. The relationship between water temperature and the development cycle beginning and duration in three black fly species. J Insect Sci 13: 1. [CrossRef] [PubMed] [Google Scholar]
- Car M, Lechthaler W. 2002. First records of Simulium (Hellichiella) latipes (Meigen) and Simulium (Simulium) ibariense Zivkovich & Grenier, Simulium codreanui (Sherban) and the occurrence of Simulium (Simulium) bezzii (Corti 1914) (Diptera, Simuliidae) in Austria. Limnologia 32: 255–272. [CrossRef] [Google Scholar]
- Chaoui Boudghane-Bendiouis C, Abdellaoui-Hassaine K, Belqat B, Franquet E, Boukli Hacene S, Yadi B. 2014. Habitat Characterization of Black Flies (Diptera: Simuliidae) in the Tafna Catchment of Western Algeria. Open J Ecol 4: 1014–1024. [CrossRef] [Google Scholar]
- Cherairia M, Adler P, Samraoui B. 2014. Biodiversity and bionomics of the black flies (Diptera: Simuliidae) of Northeastern Algeria. Zootaxa 3796: 166–174. [CrossRef] [Google Scholar]
- Chmielewski CM, Hall RJ. 1992. Responses of immature blackflies (Diptera: Simuliidae) experimental pulses of acidity. Can J Fish Aquat Sci 49: 833–840. [CrossRef] [Google Scholar]
- Ciadamidaro S, Mancini L, Rivosecchi L. 2016. Black Flies (Diptera, Simuliidae) as ecological indicators of stream ecosystem health in an urbanizing area (Rome, Italy). Ann Ist Super Sanita 52: 269–276. [PubMed] [Google Scholar]
- Ciborowski JJH, Craig DA, Fry KM. 1997. Dissolved organic matter as food for black fly larvae (Diptera:Simuliidae). J North Am Benthol Soc 16: 771–780. [CrossRef] [Google Scholar]
- Crosskey RW. 2002. A taxonomic account of the blackfly fauna of Iraq and Iran, including keys for species identification (Diptera: Simuliidae). J Nat Hist 36: 1841–1886. [CrossRef] [Google Scholar]
- Crosskey RW, Crosskey ME. 2002. An investigation of the blackfly fauna of Andalusia, southern Spain (Diptera: Simuliidae). J Nat Hist 34: 895–951. [Google Scholar]
- Crosskey RW, Howard TMA. 2004. A revised taxonomic and geographical inventory of world blackflies (Diptera: Simuliidae). Department of Entomology, Natural History Museum, London. [Google Scholar]
- Crosskey RW, Zwick H. 2007. New Faunal records with taxonomic annotations for the black flies of Turkey (Diptera: Simuliidae). Aquat Insects 29: 21–48. [CrossRef] [Google Scholar]
- CSN 75 7716. 1998. Water quality, biological analysis, determination of saprobic index. Czech Technical State Standard. Prague: Czech Standards Institute. [Google Scholar]
- Cummins KW. 1987. The functional role of black flies in stream ecosystems. In: Kim KC, Merrit RW. (eds), Black Flies: Ecology, Population Management and Annotated World List (pp. 1–10). Pennsylvania State University Press. [Google Scholar]
- Currie DC, Adler PH. 2008. Global diversity of black flies (Diptera: Simuliidae) in freshwater. Hydrobiologia 595: 469–475. [CrossRef] [Google Scholar]
- Davies TD, Tranter M, Blackwood IL, Abrahams PW. 1993. The character and causes of a pronounced snowmelt-induced ‘acidic episode’ in a stream in a Scottish sub-Arctic catchment. J Hydrol 146: 267–300. [CrossRef] [Google Scholar]
- De Pauw N, Gabriels W, Goethals PLM. 2006. River monitoring and assessment methods based on macroinvertebrates. In: Ziglio, G., Siligardi, M., Flaim, G. (eds.), Biological Monitoring of Rivers, Applications and Perspectives. Chichester: John Wiley & Sons, pp. 113–134. [Google Scholar]
- Feld CK, Kiel E, Lautenschlager M. 2002. The indication of morphological degradation of streams and rivers using Simuliidae. Limnologica 32: 273–288. [CrossRef] [Google Scholar]
- Feld CK. 2005. Assessing hydromorphological degradation of sand-bottom lowland rivers in Central Europe using benthic macroinvertebrates, Doctoral Thesis, University of Duisburg-Essen, 142 pp. [Google Scholar]
- Gallardo-Mayenco A, Toja J. 2002. Spatio-temporal distribution of simuliids (Diptera) and associated environmental factors in two Mediterranean basins of southern Spain. Limnetica 21: 47–57. [CrossRef] [Google Scholar]
- HACH. 2005. DR/890 Datalogging colorimeter handbook procedures manual, 616 p. [Google Scholar]
- Hamada NJ, McCreadie JW, Adler PH. 2002. Species richness and spatial distribution of blackflies (Diptera: Simuliidae) in streams of Central Amazonia, Brazil. Freshw Biol 47: 31–40. [CrossRef] [Google Scholar]
- Helmer R, Hespanhol I. 1997. Water Pollution Control: A Guide to the Use of Water Quality Management Principles. 526 pages. [Google Scholar]
- Hershey AE, Merritt RW, Miller MC, McCrea JS. 1996. Organic matter processing by larval black flies in a temperate woodland stream. Oikos 75: 524–532. [CrossRef] [Google Scholar]
- Hrdina A, Romportl D. 2017. Evaluating global biodiversity hotspots − very rich and even more endangered. J Landsc Ecol 10: 108–115. [CrossRef] [Google Scholar]
- Hynes HBN. 1970. The Ecology of Running Waters. Better Biological Monitoring. Liverpool University Press, 580 pp. [Google Scholar]
- Illesova D, Halgos J. 2004. Blackflies (Diptera: Simuliidae) of the Turiec River. Acta Zool Univer Comen 46: 59–63. [Google Scholar]
- Illesova D, Halgos J, Krno I. 2008. Blackfly assemblages (Diptera, Simuliidae) of the Carpathian river: habitat characteristics, longitudinal zonation, and eutrophication. Hydrobiologia 598: 163–174. [CrossRef] [Google Scholar]
- Jedlicka L, Kudela M, Stloukalova V. 2004. Key to the identification of blackfly pupae (Diptera: Simuliidae) of Central Europe. Biol Bratisl 59: 157–178. [Google Scholar]
- Jensen F. 1997. Diptera Simuliidae, Blackflies, in: Nilsson A.N. (ed.), Aquatic Insects of North Europe (pp. 209–241). [Google Scholar]
- Kachvoryan EA, Oganesyan VS, Petrova NA, Zelentsov NI. 2007. The fauna of chironomids and blackflies (Diptera: Chironomidae, Simuliidae) and hydrochemical characteristics of the Hrazdan River (Armenia). Entomol Rev 87: 73–81. [CrossRef] [Google Scholar]
- Kazancı N. 2006. Ordination of Simuliidae and climate change impact. Acta Entomol Serb (Suppl.), 69–76. [Google Scholar]
- Kazancı N. 2009. Records of Plecoptera (Insecta) species and effects of episodic acidification on physico-chemical properties of their habitats in the Eastern Black Sea Region and Yeşilırmak River Basin (Turkey). Rev Hydrobiol 2: 197–206. [Google Scholar]
- Kazancı N, Ertunç Ö. 2008. On the Simuliidae (Insecta, Diptera) Fauna of Turkey (in Turkish). Rev Hydrobiol 1: 27–36. [Google Scholar]
- Kazancı N, Ertunç Ö. 2010. Use of Simuliidae (Insecta, Diptera) species as indicators of aquatic habitat quality of Yeşilırmak River Basin (Turkey). Rev Hydrobiol 3: 27–36. [Google Scholar]
- Kazancı N, Öz B, Türkmen G, Ertunç Başören Ö. 2011. Contributions to aquatic fauna of a Biodiversity Hotspot in Eastern Black Sea Region of Turkey with records from running water interstitial fauna. Rev Hydrobiol 4: 131–138. [Google Scholar]
- Kiel E. 2001. Behavioural response of blackfly larvae (Simuliidae, Diptera) to different current velocities. Limnologica 31: 179–183. [CrossRef] [Google Scholar]
- Klee O. 1991. Angewandte Hydrobiologie. Georg Thieme Verlag Stuttgart - New York. [Google Scholar]
- Lampert W, Sommer U. 2007. Limnoecology: The Ecology of Lakes and Streams. New York: Oxford University Press. [Google Scholar]
- Landeiro VL, Pepinelli M, Hamada N. 2009. Species richness and distribution of black flies (Diptera: Simuliidae) in the Chapada Diamantina region, Bahia, Brazil. Neotrop Entomol 38: 332–339. [CrossRef] [PubMed] [Google Scholar]
- Lautenschlager M, Kiel E. 2005. Assessing morphological degradation in running waters using blackfly communities (Diptera, Simuliidae): Can habitat quality be predicted from land use? Limnologica 35: 262–273. [CrossRef] [Google Scholar]
- Lechthaler W, Car M. 2005. Simuliidae − Key to Larvae and Pupae from Central and Western Europe. CD-Rom − Edition, Vienna. [Google Scholar]
- Lechthaler W, Moog O, Car M. 2017. Diptera: Simuliidae. In Moog, O., & Hartmann, A. (Eds.) Fauna Aquatica Austriaca. BMLFUW: Wien. [Google Scholar]
- Lock K, Adriaens T, Goethals P. 2014. Effect of water quality on black flies (Diptera: Simuliidae) in Flanders (Belgium). Limnologica 44: 58–65. [CrossRef] [Google Scholar]
- Lopez-Pena D, Garcia-Roger EM, Jimenez-Peydro R. 2020. Pre-imaginal black fly assemblages in streams of Eastern Spain: environmental and substrate requirements. Hydrobiologia 847: 1521–1538. [CrossRef] [Google Scholar]
- Lopez-Pena D, Jimenez-Peydro R. 2020. Biodiversity of blackflies (Diptera, Simuliidae) in the basins of the Algar, Amadorio, Monnegre and Serpis rivers (Alicante and Valencia Provinces, East of the Iberian Peninsula). Bol Asoc Esp Entomol 44: 379–400. [Google Scholar]
- Lounaci A, Brosse S, Thomas A, Lek S. 2000. Abundance, diversity and community structure of macro- invertebrates in an Algerian stream: the Sebaou wadi. Ann Limnol 36: 123–133. [CrossRef] [EDP Sciences] [Google Scholar]
- Malmqvist B, Zhang Y, Adler PH. 1999. Diversity, distribution, and larval habitats of North Swedish blackflies (Diptera: Simuliidae). Freshw Biol 42: 301–314. [CrossRef] [Google Scholar]
- McCreadie JW, Adler PH, Hamada N, Grillet ME. 2006. Sampling and statistics in understanding distributions of black fly larvae (Diptera: Simuliidae). Acta Entomol. Serb. (Suppl.) 89–96. [Google Scholar]
- Morrison G, Fatoki OS, Persson L, Ekberg A. 2001. Assessment of the impact of point source pollution from the Keiskammahoek Sewage Treatment Plant on the Keiskamma River − pH, electrical conductivity, oxygen-demanding substance (COD) and nutrients. Water SA 27: 475–480. [CrossRef] [Google Scholar]
- Murray-Bligh JAD. 1999. Procedures for collecting and analysing macroinvertebrate samples. Quality Management Systems for Environmental monitoring: Biological Techniques, BT001. Version 2.0, Bristol, Environment Agency. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [PubMed] [Google Scholar]
- Ofenböck T, Moog O, Car M. 2002. Do the Austrian blackfly fauna (Diptera: Simuliidae) support the typological approach of the EU water framework directive? Limnologica 32: 255–272. [CrossRef] [Google Scholar]
- Parkes AH, Kalff J, Boisvert J, Cabana G. 2004. Feeding by black fly (Diptera:Simuliidae) larvae causes downstream losses in phytoplankton, but not bacteria. J North Am Benthol Soc 23: 780–792. [CrossRef] [Google Scholar]
- Rabha B, Dhiman S, Yadav K, Hazarika S, Bhola RK, Veer V. 2013. Influence of water physicochemical characteristics on Simuliidae (Diptera) prevalence in some streams of Meghalaya, India. J Vector Borne Dis 50: 18–23. [PubMed] [Google Scholar]
- Rosenberg DM, Resh VH. 1993. Freshwater biomonitoring and benthic invertebrates. New York: Chapman and Hall. [Google Scholar]
- Rubtsov IA. 1990. Black flies (Simuliidae), Fauna of the USSR Diptera Volume 6, Part 6, Brill Academic Publisher. [Google Scholar]
- Scheder C, Waringer JA. 2002. Distribution patterns and habitat characterization of Simuliidae (Insecta: Diptera) in a low-order sandstone stream (Weidlingbach, Lower Austria). Limnologica 32: 236–247. [CrossRef] [Google Scholar]
- Scheder C. 2004. The National Park Kalkalpen as a refuge area for rare species: Simulium (Obuchovia) auricoma and Simulium (Simulium) degrangei − recorded for the first time in Upper Austria. Acta Zool Univers Comen 45: 31–37. [Google Scholar]
- Schmedtje U, Colling M. 1996. Ökologische Typisierung der aquatischen Makrofauna. Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft 96: 543. [Google Scholar]
- Seitz G. 1992. Verbreitung und Okologie der Kriebelmucken (Diptera: Simuliidae) in Niederbayern. Lauterbornia 11: 1–231. [Google Scholar]
- Seitz G. 1994. Neue und bemerkenswerte Kriebelmückenfunde (Diptera: Simuliidae) für die deutsche Fauna. Lauterbornia 15: 101–109. [Google Scholar]
- Sensoy S, Demircan M, Ulupınar U, Balta I. 2008. Turkey Climate. https://www.mgm.gov.tr/FILES/genel/makale/13_turkiye_iklimi.pdf [Google Scholar]
- Stangler A, Halgos J. 2007. The structure of blackfly communities (Diptera, Simuliidae) in the longitudinal profile of some streams in the Male Karpaty Mts and the Borska nizina lowland. Acta Zool Univers Comen 47: 237–245. [Google Scholar]
- Strangler A, Halgos J, Beracko P. 2013. Blackfly (Diptera, Simuliidae) communities and species richness estimation in Carpathian montane streams. Cent Eur J Biol 8: 681–692. [Google Scholar]
- Suzuki K. 1982. Chemical changes of snow cover by melting. Jpn J Limnol 43: 102–112. [CrossRef] [Google Scholar]
- Talbalaghi A, Pessino M, Agosta P, Bo T, Ignjatovic-Cupina A. 2006. Overview of possible Simuliidae related problems in the Alessandria district (Piedmont, Italy). Acta Entomol Serb (Suppl.) 77–81. [Google Scholar]
- Ter Braak CJF. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179. [Google Scholar]
- Ter Braak CJF, Smilauer P. 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Microcomputer Power (Ithaca NY, USA). [Google Scholar]
- TSWQR. 2015. Turkish surface water quality regulation. The Official Gazette. No: 29327, Turkey (in Turkish). [Google Scholar]
- Vaananen R, Nieminen M, Vuollekoski M, Ilvesniemi H. 2006. Retention of phosphorus in soil and vegetation of a buffer zone area during snowmelt peak flow in southern Finland. Water Air Soil Pollut 177: 103–118. [CrossRef] [Google Scholar]
- Wellington BI, Driscoll CT. 2004. The episodic acidification of a stream with elevated concentrations of dissolved organic carbon. Hydrol Process 18: 2663–2680. [CrossRef] [Google Scholar]
- Werner D. 2008. 4.3.25 Simuliidae. In Ziegler, J. (Eds), Diptera Stelviana. Studia dipterologica (Supp.) 16: 297–304. Ampyx-Verlag [Google Scholar]
- Wright JF. 2000. An introduction to RIVPACS. In: Wright J. F., Sutcliffe D. W. and Furse M.T. (eds), Assessing the biological quality of fresh waters. RIVPACS and other techniques. Freshwater Biological Association, FBA Special Publications, 1–24. [Google Scholar]
- Wotton RS. 2009. Feeding in blackfly larvae (Diptera; Simuliidae) − the capture of colloids. Acta Zool Lituan 19: 64–67. [CrossRef] [Google Scholar]
- Zhang Y, Malmqvist B, Englund G. 1998. Ecological processes affecting community structure of blackfly larvae in regulated and unregulated rivers: a regional study. J Appl Ecol 35: 673–686. [CrossRef] [Google Scholar]
Cite this article as: Başören Ö, Kazancı N. 2022. The Effects of Environmental Variables on the Distribution of Immature Black Flies (Diptera, Simuliidae) in Various Streams of Northeastern Turkey. Int. J. Lim. 58: 14:
All Tables
Environmental characteristics and water quality classes of the 41 studied sites. Abbreviations: SZ: Stream Zone, CV: Current Velocity, WT: Water Temperature, EC: Electrical Conductivity, DO: Dissolved Oxygen, FWQ: Final Water Quality Classes. *Sites with episodic acidification.
All Figures
 |
Fig. 1 Study area in Eastern Black Sea Region. (I: Yeşilırmak River (Sites 1, 2, 3), II: Kelkit Stream (Sites 24, 25, 26, 27, 28, 29, 30, 37, 38, 39, 40, 41), III: Kümbet Valley and Aksu Stream (Sites 4, 5, 31, 32, 33, 34, 35, 36), IV: Altındere Valley (Sites 6, 7), V: Çaykara Stream (Sites 8, 9), VI: Anzer Valley and İkizdere Stream (Sites 16, 17, 18, 19, 20, 21, 22, 23), VII: Ayder Valley and Fırtına Stream (Sites 13, 14, 15), VIII: Ovit Valley (Sites 10, 11, 12). |
| In the text | |
 |
Fig. 2 CCA1 diagram with selected environmental variables and Simuliidae species (•: Species, □: Sites). Abbreviations: P. tomosvaryi − P.tom; M. subalpina − M. sub; S. angustipes − S. ang; S. costatum − S. cos; S. cryophilum − S. cry; S. vernum − S. ver; S. argenteostriatum − S. arg; S. bezzi − S. bez; S. hispaniola − S. his; S. ornatum − S. orn; S. trifascitum − S. tri; S. variegatum − S. var; S. balcanicum − S. bal; S. lineatum − S. lin; S. pseudequinum − S. pse. |
| In the text | |
 |
Fig. 3 CCA2 diagram with selected environmental variables and Simuliidae species (•: Species, □: Sites). Abbreviations: P. fulvipes − P. ful; P. rufipes − P. ruf; S. angustitarse − S. ang; S. cryophilum − S. cry; S. vernum − S. ver; S. argyreatum − S. arg; S. bezzi − S. bez; S. hispaniola − S. his; S. ornatum − S. orn; S. trifascitum − S. tri; S. variegatum − S. var; S. auricoma − S. aur. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


