| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 23 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/limn/2021021 | |
| Published online | 21 October 2021 | |
Research Article
Acute Exposure to the Neonicotinoid Insecticide Imidacloprid of Zebrafish (Danio rerio) Gonads: A Histopathological Approach
Sakarya University, Science and Letters Faculty, Department of Biology, 54050 Serdivan-Sakarya, Turkey
* Corresponding author: cansua@sakarya.edu.tr
Received:
12
August
2021
Accepted:
1
October
2021
Neonicotinoids are the new class of insecticides that are high target specificity to insects. Imidacloprid is a neonicotinoid that is the most widely used insecticide in the world. As a result of its widespread use in agriculture, imidacloprid interferes with the aquatic system and threatens the aquatic environment. In this study, an investigation of the histopathological effects of imidacloprid on zebrafish gonads was aimed. Zebrafish were exposed to 9.5 mg/L, 19 mg/L, and 38 mg/L of imidacloprid for 5 days, considering the 96 h LC50 value. After dissecting the gonadal tissues, routine histological techniques were applied, and the tissues were stained with Periodic Acid-Schiff (PAS), Toluidine Blue (TB), and Haematoxylin and Eosin (H&E). Sections were examined under a light microscope. While normal gonad histology was observed in the control group, histopathological alternations such as degeneration and union in the seminiferous tubules, hypertrophy in spermatogenic and Leydig cells, and interstitial fibrosis were detected in testicular tissue of the experimental groups. In the ovarian tissues of the experimental groups, structural deterioration in oocytes, autolysis, increase in the number of atretic oocytes, vacuolization in cortical alveoli, thickening and curling in the zona radiata, and opening in the perifollicular layers were detected.
Key words: Neonicotinoid / imidacloprid / zebrafish / gonad / histopathology
© EDP Sciences, 2021
1 Introduction
Pesticides are mixtures of substances that are used to prevent, control, or reduce harmful organisms (WHO, 2019). They include a wide range of products such as herbicides, insecticides, fungicides, rodenticides, etc. Pesticides are widely used in modern agriculture and provide food security for the ever-increasing population worldwide, as they are the most economical and easiest way to grow agricultural products. By 2020, global pesticide usage has been estimated to increase up to 3.5 million tonnes (Sharma et al., 2019). Although they are beneficial in agricultural production, pesticides have harmful effects on living organisms and ecosystems because of the bio-accumulation potential(Aktar et al., 2009; Sharma et al., 2019).
Neonicotinoids are a new class of pesticides that were first introduced in the 1990s, and since then, they have become one of the most widely used class of insecticide in the world (Jeschke et al., 2011; Casida and Durkin 2013). They are synthetic derivatives of nicotine, the tobacco toxin (Honda et al., 2006). Like nicotine, neonicotinoids act on certain kinds of receptors in the nerve synapse. They agonistically bind to nicotinic acetylcholine receptors on the post-synaptic nerve membrane and show their toxicity as stimulating uncontrollable and uninterrupted impulses (Tomizawa and Yamamoto, 1992; Buckingham et al., 1997; Matsuda et al., 2001). This overstimulation case finally causes paralysis and even death (Pagano et al., 2020).
Neonicotinoids are one of the largest selling group of insecticides, including imidacloprid, acetamiprid, thiacloprid, dinotefuran, nitenpyram, thiamethoxam, and clothianidin (Gupta and Milatovic, 2014; Maloney et al., 2017). They are highly water-soluble chemicals that break down slowly in the environment, contaminate global surface waters and have toxic effects on aquatic organisms (Morrissey et al., 2015).
Imidacloprid [1-[(6-chloro-3-pyridiny1)methyl]-N-nitro-2-imidazolidinimine] is the first representative of the neonicotinoid insecticides and was discovered in 1984 by chemists at Nihon Bayer Agrochem (Shiokawa et al., 1986). Imidacloprid is highly absorbed and primarily metabolized in the liver and excreted via urine when administered orally (Sheets, 2010). Because imidacloprid is frequently used in agriculture, it is involved in the aquatic ecosystem through groundwater with storms, run-off, and spray drift, and threatens the health of aquatic organisms (Gupta et al., 2002; Weston et al., 2015). Thus, the potential adverse effects of imidacloprid exposure on aquatic organisms have been of great interest to researchers. Although studies on the neurotoxicity and developmental toxicity of imidacloprid in fish are available, studies on reproductive toxicity are limited (Tisler et al., 2009; Wu et al., 2018; Vignet et al., 2019). Zebrafish are a highly preferred vertebrate model in ecotoxicological studies because they are easy to breed in laboratory conditions, become adults in 3–4 months, and are resistant to changing environmental conditions. Zebrafish, used in all areas of environmental toxicology, is used extensively as a tool in the qualitative or quantitative screening of wastewater for effective levels of toxic substances, particularly in tests to monitor toxic potential in specific environments (Stegeman et al., 2010). This study aimed to observe the histopathological effects of imidacloprid on the gonadal tissue of zebrafish.
2 Materials and methods
2.1 Test chemical
Imidacloprid (CAS No: 138261-41-3) was purchased from Sigma Aldrich (Germany).
2.2 Animal husbandry and experimental design
Adult zebrafish were obtained from Sakarya University Aquaculture Lab., Esentepe, Sakarya, Turkey. They were maintained under standardized laboratory conditions (28.5 ± 1 °C temperature, 14 h light/10 h dark photoperiod, 7.0 ± 0.5 pH, and 6.0 mg/L dissolved oxygen). Zebrafish were fed with an artificial diet TetraMin© Haupt-futter (Tetra Werke, Germany) twice a day.
The fish were randomly divided into four groups (one control and three exposure groups) and each containing 10 individuals (5 female and 5 male). While the control group just received dechlorinated tap water, exposure groups received 9.5 mg/L, 19 mg/L and 38 mg/L of imidacloprid according to 96 h LC50 value (76.08 mg/L) (Wu et al., 2018), exposure concentrations were determined. During the exposure, mortality was monitored.
2.3 Histology
After five days of exposure, the ovary and testis were dissected to investigate the effects of imidacloprid. Tissues were fixed with Bouin's fixative for 22 hours. For dehydration, tissues were passed through the ascending series of ethyl alcohol (%70, %90 and %100 EtOH). After dehydration, tissues were cleared with xylene and embedded in paraffin. Tissues were cut into 5 μm sections with a microtome (Leica, Germany). They were stained with Periodic Acid-Schiff (PAS), Toluidine Blue (TB), and Hematoxylin and Eosin (H&E). Slides were examined under a compound microscope (Leica DM 500). All experiments were performed in triplicates.
2.4 Semiquantitative scoring
A semiquantitative scoring was performed according to Mishra and Mohanty (2008). Three individuals were randomly selected from each group, and ten slides were investigated per each. The histomorphological changes were categorized as none, mild (25% of sections), moderate (25–50% of sections), and severe (>50% of sections).
3 Results
As a result of the histological evaluation, it was concluded that the exposure of imidacloprid caused damage to the reproductive organs of female and male zebrafish. The general histopathological lesions observed according to the exposure concentrations are given in Table 1 by semiquantitative scoring as mild, moderate, and severe.
Mortality was not observed in any group during the experiment. In the control group, normal gonad histology was examined. Primary, cortical alveolar, vitellogenic, and mature oocyte types were easily noticed in the ovary. A proportional increase in follicle size was observed at each stage of development. A wide range of interstitial tissue was also detected between the oocytes. In the first stage of development, primary oocytes were small in size, large in nucleus, and oval in shape. Numerous nucleoli were observed in the nucleus periphery. In cortical alveolar oocytes, granules called cortical alveoli began to appear around the ooplasm. As the oocyte grows, the cortical alveoli fill the entire ooplasm. The formation of perifollicular layers (zona radiata and follicular epithelium) around these oocytes was observed. In the vitellogenic stage, vitellogenesis was detected in the ooplasm. Yolk accumulation started from the center of the ooplasm to the periphery. These vitellus droplets began to spread around the oocyte and replace the cortical alveoli. In mature oocytes, it was observed that the follicle volume reached the maximum size, and the ooplasm was filled with yolk granules (Fig. 1a, 1b). In testicular tissue, spermatogenic cells within the seminiferous tubules were monitored clearly. Interstitial tissue with Leydig cells was detected between tubules. In seminiferous tubules, pyramid-shaped Sertoli cells were lying within the seminiferous epithelium. Spermatogonia, the largest spermatogenic cell, with pale nucleus were apparent in the base of seminiferous tubules. Many primary and secondary spermatocytes were intermediate in size with dense nuclei were detected in different meiosis phases. Spermatids derived from spermatocytes were detected with eosinophilic cytoplasm and dense nuclei. Sperms, the smallest spermatogenic cells, were observed with dark and round nuclei with no apparent cytoplasm (Fig. 1c, 1d)
In the 9.5 mg/L of imidacloprid exposed group, granulomatous inflammation was evident in ovary (Fig. 2a). Histomorphological changes such as openings at perifollicular layers (Fig. 2a, 2c) and fluctuation at zona radiata were observed (Fig. 2b). In some oocytes, zona radiata layer was thickened (Fig. 2b). Distortions in oocyte shape were apparent in many oocytes (Fig. 2b). Vacuolization and unification in cortical alveoli, which is a sign of autolysis, were detected (Fig. 2c). In the ovary, hyperplastic perifollicular cells was also monitored (Fig. 2a, 2b). In testis, the proportion of spermatogenic cells were average. Degeneration and union at seminiferous tubules were not detected. Mild histopathological changes were observed. Interstitial fibrosis was visualized between seminiferous tubules (Fig. 2d). Vascular congestion was evident in the interstitial tissue (Fig. 2e). Hypertrophy at spermatogonia and Leydig cells was monitored (Fig. 2e, 2f).
In the 19 mg/L of imidacloprid exposed group, it was noted that histopathological effects were significantly increased when compared with the control and 9.5 mg/L imidacloprid exposed groups. An increase in the number of atretic oocytes was observed in the ovarian tissue (Fig. 3a). Distortions in oocyte morphology were much more pronounced. Openings at perifollicular layers were also detected in this group (Fig. 3b). Vacuolization and unification in cortical alveoli were clearly monitored in many oocytes. Yolk granules were either degenerated or disappeared (Fig. 3b, 3c, 3d). Besides, differentiation in nucleus morphology, distortions of karyoplasm, disruption of nucleolus organization, and karyorrhexis were detected in oocytes (Fig. 3c, 3d). Degeneration was detected in the seminiferous tubules in the testicular tissue. It was observed that the boundaries of many tubules disappeared (Fig. 3e). Unification in some tubules was noted (Fig. 3e, 3f). In some seminiferous tubules, sperm clusters were detected, while other spermatogenic cells were disappeared. Moreover, the enlarged interstitial tissue was remarkable (Fig. 3f). Hypertrophic spermatogonia (Fig. 3g) and edema in seminiferous tubule were detected (Fig. 3h).
It was noted that the gonadal histopathological damage was quite severe in the 38 mg/L of imidacloprid exposed group. In ovarian tissues, an increase in the number of atretic oocytes was remarkable. Distortions in oocyte morphology, seen in other exposure groups, were also evident in this group. In some oocytes, thickening of the zona radiata layer was detected (data not shown). Degenerations of nucleus morphology were detected (Fig. 4a, 4b). Karyorrhexis was monitored when the nuclei of some oocytes were examined (Fig. 4a). It was determined that the nucleolus organization was disrupted, and some nucleoli were clustered in the nucleus (Fig. 4b). Severe vacuolization and unification were severe in cortical alveoli with signs of autolysis (Fig. 4c). Perifollicular layer openings were detected in many oocytes (Fig. 4c, 4d). In mature oocytes, the integrity of the ooplasm was impaired. Hyperplasia of the perifollicular cells was also monitored. Due to the increase in imidacloprid concentration, many developed oocytes have been observed to undergo atresia (Fig. 4d). In testis, unification at many seminiferous tubules was apparent (Fig. 4e, 4g). Fluctuations were observed at the borders of some seminiferous tubules (Fig. 4f). Severe hypertrophy was detected in spermatogenic cells like spermatogonia (Fig. 4e) and primary spermatocytes (Fig. 4g). Moreover, severe hypertrophy of Leydig cells, located between the seminiferous tubules and responsible for hormone production, was also observed (Fig. 4h).
Semiquantitative scoring of testis and ovary of zebrafish, which exposed to different concentrations (9.5, 19, and 38 mg/L) of imidacloprid. Histopathological lesions were scored to their severity (−: none, +: mild, ++: moderate, +++: severe).
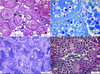 |
Fig. 1 Gonad histology of the control group, a) General view of the ovary tissue and different stages of oocytes, H&E stain, b) Zona radiata layer and surrounding sheath of perifollicular cells, black arrow: vitelline envelope, red arrow: zona radiata, yellow arrow: perifollicular cells, TB stain, c) Overview of seminiferous tubules, PAS stain, d) Spermatogenic cells in the seminiferous tubule, red arrow: spermatogonia A, green arrow: spermatogonia B, White arrow: Sertoli cell, H&E stain, Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Vo: Vitellogenic oocyte, Mo: Mature oocyte, IT: Interstitial tissue, T: seminiferous tubule, LC: Leydig cells, SG: spermatogonia, PST: primary spermatocyte, SST: secondary spermatocyte, STD: spermatids, S: sperms. |
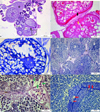 |
Fig. 2 Gonad histology of 9.5 mg/L of imidacloprid exposed group, a) Granulomatous inflammation (star), hyperplasia at perifollicular cells (arrow), and openings at follicular layers (square), PAS stain, b) distortion at oocyte shape, fluctuation at zona radiata (yellow arrow), hyperplasia at perifollicular cells in higher magnification (black arrow), H&E stain, c) vacuolization and unification in cortical alveoli (red rectangle), openings at follicular layers in higher magnification (square), TB stain, d) interstitial fibrosis (IF) between tubules, PAS stain, e) hypertrophy at Leydig cells (green arrows) and vascular congestion (red star), PAS stain, f) hypertrophy at spermatogonia (red arrows), TB stain, Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Vo: Vitellogenic oocyte, N: nucleus, No: Nucleolus, T: seminiferous tubules, SGA: spermatogonia A, SGB: spermatogonia B, PST: primary spermatocyte, SST: secondary spermatocyte. |
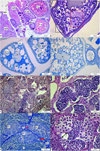 |
Fig. 3 Gonad histology of 19 mg/L of imidacloprid exposed group, a) increase in the number of atretic oocytes, H&E stain, b) vacuolization and unification at cortical alveoli, PAS stain, c) Distortions at karyoplasm and disruption of nucleolus organization, TB stain, d) Karyorrhexis at primary oocyte, TB stain, e) distortion at unification seminiferous tubules, PAS stain, f) sperm clusters in seminiferous tubules, H&E stain, g) hypertrophy of spermatogonia (red arrow), TB stain, h) edema in theseminiferous tubule (double headed arrow), H&E stain, Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Vo: Vitellogenic oocyte, Ca: cortical alveoli, AO: Atretic oocyte, IT: interstitial tissue, N: Nucleus, No: Nucleolus, T: seminiferous tubules, red rectangle: vacuolization and unification at cortical alveoli, arrow: karyorrhexis black rectangle: distortion at unification seminiferous tubules, black square: openings at perifollicular layers SG: spermatogonia, PST: primary spermatocyte, SST: secondary spermatocyte, STD: spermatid, S: sperm. |
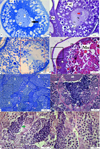 |
Fig. 4 Gonad histology of 38 mg/L of imidacloprid exposed group, a) Karyorrhexis at the nucleus of cortical alveolar stage oocyte, TB stain, b) Disruption of nucleolus organization and formation of nucleolus clusters in the center of the nucleus, PAS stain, c) Vacuolization and unification at cortical alveoli, TB stain, d) Impaired ooplasm at mature oocytes, H&E stain, e) Unification at seminiferous tubules and hypertrophic spermatogonia, TB stain, f) fluctuation at the border of seminiferous tubules, H&E stain, g) Hypertrophy at primary spermatocytes, PAS stain, h) hypertrophy at Leydig cell, PAS stain. Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Ca: cortical alveoli, Vo: Vitellogenic oocyte, Mo: Mature oocyte, N:nucleus, No: nucleolus, ZR: Zona radiata, Fe: Follicular epithelium, T: seminiferous tubule, SG: spermatogonia, PST: primary spermatocyte, SST: secondary sprmatocyte, STD: spermatid, St: Sertoli cell, LC: Leydig cell, black arrow: karyorrhexis, red arrow: clusters of the nucleolus, red rectangle: vacuolization and unification of cortical alveoli, black square: openings at perifollicular layers, black rectangle: unification of seminiferous tubules, yellow arrow: hyperplasia at perifollicular cells, star: impaired ooplasm, pink arrow: hypertrophy at spermatogonia, white arrow: fluctuations at the border of the seminiferous tubule, green arrow: hypertrophy at primary spermatocyte, asterisks: hypertrophy at Leydig cell. |
4 Discussion
Many pesticides are on the agenda of scientists with the decrease of agricultural areas, an increase of pests, and a shortening of harvest time. Pesticides intensively interfere with the aquatic ecosystems. Many pesticides are proven or suspected to be endocrine disruptors (McKinlay et al., 2008). Histology is a useful and reliable method used to investigate the toxic effects of endocrine-related chemicals of fish gonads on the tissue and cell level (OECD, 2010).
The data about the toxic effects of imidacloprid on aquatic organisms are limited. In a study conducted by Tisler et al. (2009), imidacloprid was found to be a hazardous to bacteria Vibrio fischeri, algae Desmodesmus subspicatus, crustacean Daphnia magna, and fish Danio rerio. According to Mikolić and Karačonji (2018), many pesticides, including imidacloprid, can interfere with natural hormones and affect the normal functioning of hormones. Thus imidacloprid can be included in endocrine disruptors. In this study, it was demonstrated that imidacloprid exposure caused histopathological lesions in the ovary and testis. As can be seen from the results of this study, imidacloprid acts as a reproductive toxin. This may be due to the endocrine-disrupting effect of imidacloprid.
In many studies in rats, the toxic effects on reproduction system of imidacloprid were proved by histological methods. Scientists reported that imidacloprid caused adverse histological changes on testicular tissue (Najafi et al., 2010), distortions at sperm mortality and inhibits spermatogenesis (Hafez et al., 2016), increase in the number of atretic follicles in the ovary (Kapoor et al., 2011), and significant pathomorphological changes in different stages of follicles (Kapoor et al., 2011; Vohra and Khera, 2016). Parallel to the findings of the many studies in rats, it was determined that imidacloprid also caused reproductive toxicity in the ovary and testis of zebrafish. In a study with quails, similar findings were observed, and it was determined that imidacloprid caused vacuolization and DNA fragmentation in germ cells in the seminiferous tubules of the testis (Hoshi et al., 2014).
In a study with freshwater fish Australoheros facetus, imidacloprid was found to cause genotoxic effects (Iturburu et al., 2018). In another study by Petrovici et al. (2020), it was determined that another pesticide, deltamethrin, caused cell death in germ cells in the testis and ovary. These may explain the histopathological findings pointing to autolysis observed in germ cells in this study.
While there are not many studies in the literature investigating the reproductive histo-toxic effects of imidacloprid on aquatic organisms, there are studies about other pesticides. In another study investigating the histopathological effects of β-endosulfan in the ovarian tissue of zebrafish, it was found that this substance caused folding in the oocyte membrane, increase in the number and size of follicles (Han et al., 2011). Another endocrine-disrupting chemical that has a similar effect to imidacloprid is polychlorinated biphenyl (PCB). Daouk et al. (2011) observed the effects of PCB mixtures on zebrafish. As a result of the study, they found an increase in atretic oocytes due to the increase in concentration. Koç et al. (2009) investigated the histopathological effects of deltamethrin on zebrafish ovary, and they found deltamethrin exposure inhibits oogenesis. Moreover, they observed autolysis, degenerated chromatin material, increase in the number of atretic oocytes. In a different study, Rajini et al. (2015) investigated histopathological changes in tissues of zebrafish exposed to sublethal concentration of chlorpyrifos 50% and cypermethrin 5% combination. Follicular atresia was observed in the ovary. In another study performed by Manjunatha and Philip (2016), reproductive toxicity of chlorpyrifos was observed in zebrafish. Vacuolization was detected in oocytes. Many atretic and degenerated follicles were also observed. The results of the present research are consistent with these studies. Accordingly, it can be said that imidacloprid has histo-toxic effects similar to other pesticides caused in zebrafish ovarian tissue.
Similar effects were observed not only for ovarian tissue but also for testicular tissue. Abar Gürol et al. (2020) conducted a study about the histopathological effects of mancozeb on testicular tissue of zebrafish, and they observed degenerative spermatogenic cells, seminiferous tubule disorganizations, fibrosis, hemorrhage, vacuolization, hypertrophy of spermatocytes, edema, pyknotic and karyolytic nuclei. Similar histopathological findings were also seen in this study.
In conclusion, although pesticides are produced specifically for certain taxon, the frequent use of these substances in agriculture threatens the aquatic ecosystem. It has been observed that widespread insecticide use prevents the reproduction of fish. As a result, when compared with the studies mentioned above, it can be concluded that pesticides have similar effects on fish, inhibiting the reproduction of aquatic organisms and harming germ cells.
References
- Abar Gürol M, Arman S, Yön ND. 2020. Effects of mancozeb on the testicular histology of the zebrafish (Danio rerio). Ann Limnolog Int J Limnol 56: 10. [CrossRef] [EDP Sciences] [Google Scholar]
- Aktar MW, Sengupta D, Chowdhury A. 2009. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2: 1–12. [CrossRef] [PubMed] [Google Scholar]
- Buckingham S, Lapied B, Corronc H, Sattelle F. 1997. Imidacloprid actions on insect neuronal acetylcholine receptors. J Exp Biol 200: 2685–2692. [CrossRef] [PubMed] [Google Scholar]
- Casida JE, Durkin KA. 2013. Neuroactive insecticides: targets, selectivity, resistance and secondary effects. Annu Rev Entomol 58: 99–117. [CrossRef] [PubMed] [Google Scholar]
- Daouk T, Larcher T, Roupsard F, et al. 2011. Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquat Toxicol 105: 270–278. [CrossRef] [PubMed] [Google Scholar]
- Gupta S, Gajbhiye Kalpana VT, Agnihotri NP. 2002. Leaching behavior of imidacloprid formulations in soil. Bull Environ Contam Toxicol 68: 502–508. [CrossRef] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D. 2014. Insecticides. In: Gupta RC. (ed.), Biomarkers in Toxicology, Academic Press, Cambridge, USA, 389–407. [CrossRef] [Google Scholar]
- Hafez EM, Issa SY, Al-Mazroua MK, Ibrahim KT, Rahman SMA. 2016. The neonicotinoid insecticide imidacloprid: a male reproductive system toxicity inducer-human and experimental study. Toxicol Open Access 2: 1000109. [CrossRef] [Google Scholar]
- Han Z, Jiao S, Kong D, Shan Z, Zhang X. 2011. Effects of β-endosulfan on the growth and reproduction of zebrafish (Danio rerio). Environ Toxicol Chem 30: 2525–2531. [CrossRef] [PubMed] [Google Scholar]
- Honda H, Tomizawa M, Casida JE. 2006. Insect nicotinic acetylcholine receptors: neonicotinoid binding site specificity is usually but not always conserved with varied substituents and species. J Agric Food Chem 54: 3365–3371. [CrossRef] [PubMed] [Google Scholar]
- Hoshi N, Hirano T, Omotehara T, et al. 2014. Insight into the mechanism of reproductive dysfunction caused by neonicotinoid pesticides. Biol Pharm Bull 37: 1439–1443. [CrossRef] [PubMed] [Google Scholar]
- Iturburu FG, Simoniello MF, Medici S, Panzeri AM, Menone ML. 2018. Imidacloprid Causes DNA Damage in Fish: Clastogenesis as a Mechanism of Genotoxicity. Bull Environ Contam Toxicol 100: 760–764. [CrossRef] [PubMed] [Google Scholar]
- Jeschke P, Nauen R, Schindler M, Elbert A. 2011. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59: 2897–2908. [CrossRef] [PubMed] [Google Scholar]
- Kapoor U, Srivastava MK, Srivastava LP. 2011. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol 49: 3086–3089. [CrossRef] [PubMed] [Google Scholar]
- Koç ND, Muşlu MN, Kayhan FE, Özesen Çolak S. 2009. Histopathological changes in ovaries of zebrafish (Danio rerio) following administration of deltamethrin. Fresenius Environmental Bulletin 18: 1872–1878. [Google Scholar]
- Maloney EM, Morrissey CA, Headley JV, Peru KM, Liber K. 2017. Cumulative toxicity of neonicotinoid insecticide mixtures to Chironomus dilutus under acute exposure scenarios. Environ Toxicol Chem 36: 3091–3101. [CrossRef] [PubMed] [Google Scholar]
- Manjunatha B, Philip GH. 2016. Reproductive toxicity of chlorpyrifos tested in zebrafish (Danio rerio): Histological and hormonal end points. Toxicol Ind Health 32: 1808–1816. [CrossRef] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. 2001. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22: 573–580. [CrossRef] [PubMed] [Google Scholar]
- McKinlay R, Plant JA, Bell JN, Voulvoulis N. 2008. Endocrine disrupting pesticides: implications for risk assessment. Environ Int 34: 168–183. [CrossRef] [PubMed] [Google Scholar]
- Mikolić A, Karačonji IB. 2018. Imidacloprid as reproductive toxicant and endocrine disruptor: investigations in laboratory animals. Arh Hig Rada Toksikol 69: 103–108. [CrossRef] [PubMed] [Google Scholar]
- Mishra AK, Mohanty B. 2008. Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch). Environ Toxicol Pharmacol: 136–141. [CrossRef] [PubMed] [Google Scholar]
- Morrissey CA, Mineau P, Devries JH, et al. 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74: 291–303. [CrossRef] [PubMed] [Google Scholar]
- Najafi G, Razi M, Hoshyar A, Shahmohamadloo S, Feyzi S. 2010. The effect of chronic exposure with imidacloprid insecticide on fertility in mature male rats. Int J Fertil Steril 4: 9–16. [Google Scholar]
- OECD. 2010. Guidance Document fort he Diagnosis of Endocrine-Related Histopathology of Fish Gonads. J Test Eval No. 123, 114p. [Google Scholar]
- Pagano M, Stara A, Aliko V, Faggio C. 2020. Impact of Neonicotinoids to Aquatic Invertebrates—In Vitro Studies on Mytilus galloprovincialis: A Review. J Mar Sci Eng 8: 801. [CrossRef] [Google Scholar]
- Petrovici A, Strungaru SA, Nicoara M, Robea MA, Solcan C, Faggio C. 2020. Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers. J Mar Sci Eng 8: 73. [CrossRef] [Google Scholar]
- Rajini A, Revathy K, Selvam G. 2015. Histopathological Changes in Tissues of Danio rerio Exposed to Sub Lethal Concentration of Combination Pesticide. Int J Eng Sci Technol 8: 1–12. [Google Scholar]
- Sharma A, Kumar V, Shahzad B, et al. 2019. Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1: 1446. [CrossRef] [Google Scholar]
- Shiokawa K, Tsuboi S, Kagabu S, Moriya K. 1986. Jpn. Kokai Tokkyo Koho JP 61–267575. [Google Scholar]
- Sheets LP. 2010. Imidacloprid: A Neonicotinoid Insecticide. In: Krieger R. (ed.), Hayes' Handbook of Pesticide Toxicology (Third Edition), Academic Press 1: 2055–2064. [CrossRef] [Google Scholar]
- Stegeman JJ, Goldstone JV, Hahn ME. 2010. Perspectives on zebrafish as a model in environmental toxicology. In: Perry SF, Ekker M, Farrell AP, Brauner CJ. (eds.), Fish Physiology, 29: 367–439. [CrossRef] [Google Scholar]
- Tisler T, Jemec A, Mozetic B, Trebse P. 2009. Hazard identification of imidacloprid to aquatic environment. Chemosphere 76: 907–914. [CrossRef] [PubMed] [Google Scholar]
- Tomizawa M, Yamamoto I. 1992. Binding of neonicotinoids and the related compounds to the insect nicotinic acetylcholine receptor. J Pestic Sci 17: 231–236. [CrossRef] [Google Scholar]
- Vignet C, Cappello T, Fu Q, et al. 2019. Imidacloprid induces adverse effects on fish early life stages that are more severe in Japanese medaka (Oryzias latipes) than in zebrafish (Danio rerio). Chemosphere 225: 470–478. [CrossRef] [PubMed] [Google Scholar]
- Vohra P, Khera KS. 2016. Effect of imidacloprid on reproduction on female albino rats in three generation study. J Vet Sci Technol 7: 1000340. [CrossRef] [Google Scholar]
- Weston DP, Chen D, Lydy MJ. 2015. Stormwater-related transport of the insecticides bifenthrin, fipronil, imidacloprid, and chlorpyrifos into a tidal wetland, San Francisco Bay, California. Sci Total Environ 527–528: 18–25. [CrossRef] [PubMed] [Google Scholar]
- WHO, World Health Organization. 2019. Pesticides, https://www.who.int/topics/pesticides/en/24/04/2019 [Google Scholar]
- Wu S, Li X, Liu X, et al. 2018. Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environ Pollut 235: 470–481. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Akbulut C. 2021. Acute Exposure to the Neonicotinoid Insecticide Imidacloprid of Zebrafish (Danio rerio) Gonads: A Histopathological Approach. Ann. Limnol. - Int. J. Lim. 57: 23
All Tables
Semiquantitative scoring of testis and ovary of zebrafish, which exposed to different concentrations (9.5, 19, and 38 mg/L) of imidacloprid. Histopathological lesions were scored to their severity (−: none, +: mild, ++: moderate, +++: severe).
All Figures
 |
Fig. 1 Gonad histology of the control group, a) General view of the ovary tissue and different stages of oocytes, H&E stain, b) Zona radiata layer and surrounding sheath of perifollicular cells, black arrow: vitelline envelope, red arrow: zona radiata, yellow arrow: perifollicular cells, TB stain, c) Overview of seminiferous tubules, PAS stain, d) Spermatogenic cells in the seminiferous tubule, red arrow: spermatogonia A, green arrow: spermatogonia B, White arrow: Sertoli cell, H&E stain, Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Vo: Vitellogenic oocyte, Mo: Mature oocyte, IT: Interstitial tissue, T: seminiferous tubule, LC: Leydig cells, SG: spermatogonia, PST: primary spermatocyte, SST: secondary spermatocyte, STD: spermatids, S: sperms. |
| In the text | |
 |
Fig. 2 Gonad histology of 9.5 mg/L of imidacloprid exposed group, a) Granulomatous inflammation (star), hyperplasia at perifollicular cells (arrow), and openings at follicular layers (square), PAS stain, b) distortion at oocyte shape, fluctuation at zona radiata (yellow arrow), hyperplasia at perifollicular cells in higher magnification (black arrow), H&E stain, c) vacuolization and unification in cortical alveoli (red rectangle), openings at follicular layers in higher magnification (square), TB stain, d) interstitial fibrosis (IF) between tubules, PAS stain, e) hypertrophy at Leydig cells (green arrows) and vascular congestion (red star), PAS stain, f) hypertrophy at spermatogonia (red arrows), TB stain, Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Vo: Vitellogenic oocyte, N: nucleus, No: Nucleolus, T: seminiferous tubules, SGA: spermatogonia A, SGB: spermatogonia B, PST: primary spermatocyte, SST: secondary spermatocyte. |
| In the text | |
 |
Fig. 3 Gonad histology of 19 mg/L of imidacloprid exposed group, a) increase in the number of atretic oocytes, H&E stain, b) vacuolization and unification at cortical alveoli, PAS stain, c) Distortions at karyoplasm and disruption of nucleolus organization, TB stain, d) Karyorrhexis at primary oocyte, TB stain, e) distortion at unification seminiferous tubules, PAS stain, f) sperm clusters in seminiferous tubules, H&E stain, g) hypertrophy of spermatogonia (red arrow), TB stain, h) edema in theseminiferous tubule (double headed arrow), H&E stain, Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Vo: Vitellogenic oocyte, Ca: cortical alveoli, AO: Atretic oocyte, IT: interstitial tissue, N: Nucleus, No: Nucleolus, T: seminiferous tubules, red rectangle: vacuolization and unification at cortical alveoli, arrow: karyorrhexis black rectangle: distortion at unification seminiferous tubules, black square: openings at perifollicular layers SG: spermatogonia, PST: primary spermatocyte, SST: secondary spermatocyte, STD: spermatid, S: sperm. |
| In the text | |
 |
Fig. 4 Gonad histology of 38 mg/L of imidacloprid exposed group, a) Karyorrhexis at the nucleus of cortical alveolar stage oocyte, TB stain, b) Disruption of nucleolus organization and formation of nucleolus clusters in the center of the nucleus, PAS stain, c) Vacuolization and unification at cortical alveoli, TB stain, d) Impaired ooplasm at mature oocytes, H&E stain, e) Unification at seminiferous tubules and hypertrophic spermatogonia, TB stain, f) fluctuation at the border of seminiferous tubules, H&E stain, g) Hypertrophy at primary spermatocytes, PAS stain, h) hypertrophy at Leydig cell, PAS stain. Po: Primary oocyte; CoC: Cortical alveolar stage oocyte, Ca: cortical alveoli, Vo: Vitellogenic oocyte, Mo: Mature oocyte, N:nucleus, No: nucleolus, ZR: Zona radiata, Fe: Follicular epithelium, T: seminiferous tubule, SG: spermatogonia, PST: primary spermatocyte, SST: secondary sprmatocyte, STD: spermatid, St: Sertoli cell, LC: Leydig cell, black arrow: karyorrhexis, red arrow: clusters of the nucleolus, red rectangle: vacuolization and unification of cortical alveoli, black square: openings at perifollicular layers, black rectangle: unification of seminiferous tubules, yellow arrow: hyperplasia at perifollicular cells, star: impaired ooplasm, pink arrow: hypertrophy at spermatogonia, white arrow: fluctuations at the border of the seminiferous tubule, green arrow: hypertrophy at primary spermatocyte, asterisks: hypertrophy at Leydig cell. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


