| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 22 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/limn/2021017 | |
| Published online | 21 October 2021 | |
Research Article
Effects of ethylene-bis-dithiocarbamate (Mancozeb) on zebrafish (Danio rerio) oocytes
Sakarya University, Faculty of Arts and Sciences, Department of Biology, Sakarya, Turkey
* Corresponding author: tdinc@sakarya.edu.tr
Received:
4
May
2021
Accepted:
19
September
2021
Pesticides used to protect plants and animals against the competition of unwanted insects, diseases, and weeds, and pests play a delicate role in living systems. It has adverse effects on the environment and health. The most adverse effects of pesticide derivatives are the reduction in biodiversity, water pollution, and health risks. Mancozeb is an ethylene bis dithiocarbamate (EBDC) pesticide containing manganese and zinc. These pesticides are included in the fungicide group because they are used for treating fungal diseases. In this study, the structure of oocytes developing in female zebrafish exposed to different doses of mancozeb (5 mg L−1, 7.5 mg L−1) was examined. Compared with the control group, it was determined that the number of developing oocytes reduced in the experimental groups. There was a significant increase in atretic oocytes, an increase in the level of degenerate oocytes in a dose-dependent manner, and deformation in oocytes became prominent.
Key words: Toxicology / mancozeb / ethylene-bis-dithiocarbamate / zebrafish / oocyte
© EDP Sciences, 2021
1 Introduction
Pesticides are used as chemical control agents to be economically protected from the adverse effects of diseases, pests, and weeds, which are a problem in agricultural production. The high efficiency of pesticides against harmful organisms, their rapid results, their ability to protect the product from organisms releasing toxins, and their being economical when used consciously and in a controlled manner causing them to be widely used (Karakoç and Nakiboğlu, 2010). They can threaten human and environmental health directly or indirectly because of unconscious and improper use. Nowadays, with the importance of food safety, the use of pesticides derivatives by consumers is alarming because these chemicals have some toxic effects on living things in the short or long term.
Dithiocarbamates constitute a significant class of pesticides introduced in the USA in 1962 as a broad-spectrum pesticide (EPA, 2005). It has been widely used in recent years as it is considered partially safe for mammals. Ethylene bis dithiocarbamate (EBDC) pesticides are in second place in this group of pesticides frequently applied to tomatoes in European Union countries. Mancozeb is a pesticide from the EBDC family, consisting of an EBDC and a manganese-zinc complex (Srivastava and Singh, 2013). It is used in different concentrations as the main active ingredient of some pesticides produced against pests to protect fruits in agricultural plants (Pavlovic et al., 2015). Mancozeb is hydrolyzed to metabolites such as ethylene urea (EU), ethylene bisisothi cyanate sulfide (EBIS), and ethylene thiourea (ETU) in humid environments. One of its metabolites is ETU, which translates into potential developmental, neurotoxic, and carcinogenic effects on wildlife and humans (Houeto et al., 1995; Brouwer et al., 2017; Cao et al., 2019). ETU decomposes spontaneously in the presence of water and oxygen. Its metabolite ETU has sufficient potential to contaminate groundwater (Srivastava and Singh, 2013). Mancozeb has almost low persistence in aquatic environments and is toxic to aquatic species (PPDB, 2018). According to Gullino (2010), annual sales and co-formulation of mancozeb are $750 million globally. Although mancozeb was banned in the European Parliament EP in 2009, it is still used in countries like the UK, Spain, Austria, and Denmark (PPDB, 2018).
Zebrafish are model organisms used in scientific studies. Zebrafish are preferred in many research areas such as developmental biology, genetics and ecotoxicology. Features such as low maintenance cost, short breeding cycle, rapid development, and resistance to variable environmental conditions have made this model organism more popular (Dai et al., 2014; Hoo et al., 2016; Tanguay, 2018) and have led us to prefer it in this study.
A collection of animal studies examined the effect of mancozeb exposure, a decrease in reproductive potential in female animals at higher levels of exposure. In contrast, different studies revealed that prenatal exposure to low doses of mancozeb might lead to adverse developmental toxicity (Kjeldsen et al., 2013; Runkle, 2017). Some evidence has shown lasting premalignant changes in human ovarian cells following exposure to mancozeb, elevating concerns of cancer and reproductive health risks in exposed human populations (Paro et al., 2012).
Pesticides can reach surface and ground waters due to water runoff, soil leaching, and spraying applications (Pimentel, 2004). Moreover, water contamination raises enormous concern, considering the extensive range of non-target organisms that might end up endangered. In particular, fish could be notably affected, considering that dissolved pesticides might be taken up through gills, skin, or gastrointestinal tract (Bisson, 2002). Mancozeb exposure is considered highly toxic to aquatic organisms, including fish (Maltby, 2009). The endocrine system of fish has differences compared to mammals, yet the reproductive endocrine system is similar in fish and mammals. Hence, hormones that affect the endocrine system of fish can be considered as potential endocrine disruptors in mammals, including humans (Di Giulio, 2008). In this context, it is crucial to examine the ovary, one of the reproductive organs, to reveal the potential endocrine-disrupting effect. Therefore, this study aimed to examine the histopathological effects of mancozeb on zebrafish (Danio rerio) ovarian tissue.
2 Materials and methods
2.1 Animal husbandry
In this study, a total of sexually mature female zebrafish (4.5 cm in length, approximately 2 g in weight) were used. Zebrafish individuals were kept in an aquarium of 10 × 20 ×35 cm and 20 L capacity. 24-hour dechlorinated water in the aquariums was fixed at 26–28 °C (pH 7.7, dissolved oxygen 7.9 mg L−1). The aquariums were kept in the photoperiod of 14 h light/10 h dark.
2.2 Exposure and the experimental design
Mancozeb (Dikozin, M 45, CAS 8018-01-7) was obtained from the farm supplier (Agrofarm, Istanbul) and dissolved in water to form a stock solution. Within the scope of the experiment, a total of 3 groups (n: 10) were formed as a control group and two experimental groups (5 mg L−1 and 7.5 mg L−1). After five days of exposure, the fish were euthanized in ice-cold water, and ovary tissues were dissected. The experiment was carried out in triplicate.
2.3 Histology
The ovarian tissues were kept in Bouin's fixative for 24 hours, and fixation procedures were completed. After passing through a series of ascending alcohol (70%, 80%, 90%, 96%, and 100%), the tissues were enclosed in xylene for the clearing step. Tissues were embedded in paraffin, and after that, sections of 5 μm thickness were taken with a Leica RM2125RT microtome. Ovarian tissues which went through routine histological procedures were stained with Ehrlich's Hematoxylin-Eosin (HE), Masson Trichrome, and Periodic Acid Schiff (PAS). The Leica DM500 was examined with a light microscope, and figures were taken with the Leica MC170 HD camera.
3 Results
Oocytes at different developmental stages (primary oocyte, cortical alveolar oocyte, vitellogenic oocyte, mature oocyte) were determined in the ovarian tissue of the zebrafish control group. In the primary oocyte stage, a large nucleus structure compared to the cytoplasm was observed. This is a characteristic feature for primary oocytes. Oocyte diameter increased in the cortical alveolar stage. In the vitellogenic stage, an increase in oocyte diameter was found depending on the progress of development. At this stage, the zona radiata was distinctively selected around the oocyte with its striped structure. It was identified that the nucleus was deformed in shape, and its membrane was disrupted during the maturation stage (Fig. 1A and B).
Mild histopathological changes were observed in the 5 mg L−1 mancozeb group. A decrease in the number of primary and cortical alveolar oocytes was detected. Gaps were observed between the nucleoplasm (karyoplasm) and ooplasm. Nucleoplasm shrinkage was detected (Fig. 2A, B, C, D). Morphological deformations were noted in primary oocytes, cortical alveolar oocytes, and vitellogenic oocytes. In some oocytes, discharges were observed in the areas where they were completely positioned due to atresia (Fig. 2A). Vacuolization was observed in some parts of the ooplasm. An opening was observed between the follicular epithelium and the zona radiata (Fig. 2A, C, D). Compared to the control group, an increase in the number of atretic oocytes was also detected. A reduction in connective tissue was noticed (Fig. 2A, D). In the first stage of development, complete loss of oocyte integrity was observed in some oocytes. In addition, it was determined that this exposure concentration inhibited oocytes, especially in the first stage of development. It was observed that oocytes in the later stages of development tended to atretic oocyte appearance (Fig. 2B).
Severe histopathological changes were observed in the 7.5 mg L−1 mancozeb group due to an increase in concentration. It was determined that the number of primary, cortical alveolar, and vitellogenic oocytes was also decreased. A significant increase in connective tissue was identified. It was noted that deteriorated and fragmented zona radiata in some oocytes. Separation was monitored between the vitelline envelope and zona radiata in vitellogenic oocytes (Fig. 3A). Distortions such as swelling and fusion that occurred in the cortical alveoli of vitellogenic oocytes were observed. Also, shrinkage and deterioration were noticed in the karyoplasm of vitellogenic oocytes (Fig. 3A, C). In some oocytes, it was determined that there was a membrane folding in the zona radiata (Fig. 3B).
 |
Fig. 1 Ovarian tissue of control group. PO: primary oocyte, CAO: cortical alveolar stage oocyte, VO: vitellogenic oocyte, M: mature oocyte, O: ooplasm, Kp: karyoplasm, ZR: zona radiata, A, B: H&E staining. |
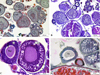 |
Fig. 2 Ovarian tissue of 5 mg L−1 mancozeb exposed group, PO: primary oocyte, CAO: cortical alveolar stage oocyte, M: mature oocyte, AT: atretic oocyte, O: ooplasm, Kp: karyoplasm, ZR: zona radiata, FE: follicular epithelium, asteriks: detachment at epithelium, arrow: shrinkage at karyoplasm, A,D: Masson Trichrome, B: PAS, C: H&E staining. |
 |
Fig. 3 Ovarian tissue of 7.5 mg L−1 mancozeb exposed group, CAO: cortical alveolar stage oocyte, VO: vitellogenic oocyte, M: mature oocyte, Ct: connective tissue, N: nucleus, Kp: karyoplasm, ZR: zona radiata, Fe: follicular epithelium, arrow: shrinking karyoplasm, square: vacuolization at cortical alveoli, yellow rectangle: deteriorated and fragmented in the zona radiata, red rectangle: separation between vitelline envelope and zona radiata, A,B: PAS, C: H&E staining. |
4 Discussion
Industrial developments have caused many organic pollutants to enter our lives as in the whole world. The random and unconscious use of pollutants such as pesticides also causes us to face many problems in the ecosystem. Although they are generally used in doses that are not harmful in practice to humans and the environment, harmful effects are seen on living things.
These results suggest that histopathological changes were observed in the ovarian tissues on the zebrafish, which was conducted to demonstrate the effects of mancozeb, a pesticide product. It was determined that this pesticide derivative negatively affected ovarian tissue and oocyte development. Morphological deformations in primary oocytes, cortical alveolar oocytes, and vitellogenic oocytes, vacuolization in some regions of the ooplasm, the separation between a follicle epithelium and zona radiata, clumping in the karyoplasm and nucleus, and an increase in the number of atretic oocytes occurred.
Mancozeb has been shown in many studies to cause adverse health effects in both humans and laboratory animals. High doses of mancozeb caused general toxicity and neurotoxic effects in rats (Kackar et al., 1997; Trivedi et al., 1993). As a result of mancozeb administration to rats, a decrease in the ovary, testis, epididymis weight, and histopathological changes in gonads were observed (Baligar and Kaliwal, 2001; Kackar et al., 1997; Mahadevaswami et al., 2000).
It has also been shown by Haider and Imbaraj (1988) that endosulfan and malathion inhibit LH-induced oocyte maturation in Cyprinus carpio. In a study conducted by Pandey (1988), when endosulfan was applied to the ovaries of the freshwater fish Colisa fasciatus, it delayed ovarian activity and significantly decreased oocyte diameter. In a study by Dutta et al. (1994), Heteropneustes fossilis were exposed with malathion (1.2 mg L−1). As a result of the study, it was stated that there was degeneration in the ovary, tearing in the follicle epithelium, clumping in the cytoplasm, and an increase in the number of atretic oocytes. The findings in this study conducted with malathion are like those in our study. In another study, H. fossilis was exposed to carbofuran, and degeneration of the follicle wall and vacuolization in the plasma of oocytes were reported (Chatterjee et al., 1997). This study supports the results we observed in our study. Channa orientalis were exposed to the organophosphorus pesticides Nuvan (0.27 mg L−1) and Dimecron (0.55 mg L−1) for a period of 30 to 45 days. In the ovary, a decrease in oocyte cap, a decrease in oocyte development stages, and an increase in the percentage of atretic follicles have been reported (Saksena, 1999). This study is similar to our study in terms of the increase in atretic follicles.
In a study, it was found that when Rasbora daniconius was administered 0.1 mg/L carbamate carbofuran and 0.001 mg/L endosulfan, ovarian tissue degeneration occurred (Rastogi and Kulshrestha, 1990; Kogan et al., 2000). It has been reported that C. punctatus ovary exposed to carbofuran has degeneration, ooplasmic fragmentation and clumping (Ram et al., 2001). These findings are also consistent with the results of our study.
In another study by Dutta et al. (2008), Lepomis macrochirus was exposed to 1 μg/l endosulfan at 25%, 75%, 100% concentration. In the histological examinations performed after 24 hours. It has been reported that adhesion between follicles, nuclear retraction, cytoplasmic retraction, clumping in the karyoplasm, and an increase in the number of atretic oocytes have been reported. In another study, zebrafish exposed to different doses of deltamethrin causes degeneration in ovarian tissue. It has been stated that deltamethrin chemical causes the emergence of morphological changes such as a reduction in primary oocytes in ovarian tissue, chromatin irregularities, degeneration in the nucleolus structure and deformation in the ooplasm, reduction in cortical alveoli numbers, irregularities in nuclei, and openings in the nucleus membrane (Koc et al., 2009). It has been stated in our study that similar effects were caused by the mancozeb.
In a study, 4 and 15 days of application of malathion 0.8 mg L−1 was applied to C. punctatus species. While a reduction in size in mature oocytes and vacuolization in the cytoplasm were found in the 4-day dose group; In the 15-day dose group, disrupted oocytes, necrosis and elongated follicles were detected (Magar et al., 2013). Findings in this study are partially consistent with our findings. In another study investigating the effects of monocrotophos, one of the organophosphorus pesticides, on the ovaries of C. punctatus, it was stated that, unlike the control group, a decrease in vitellogenesis and atresia was observed after exposure to each concentration for 15 days. After 45 days of exposure, significant vacuolization findings and an increase in oocyte atresia accompanying tissue necrosis, and signs of reduced vitellogenesis were reported (Maqbool and Ahmed, 2013). The findings of this study are consistent with the results we obtained.
In another study, fish of the Oreochromis mossambicus were exposed to mancozeb. In this study, excessive mucus secretion on all body surfaces of fish, possibly due to high toxic substances, was observed in a state of extreme stress (Saha et al., 2016). It has been determined that the survival rate of P. ticto decreases due to the increase in mancozeb concentration (Sharma et al., 2016). In a study by Luzio et al. (2016), 17-ethinylestradiol and fadrozole that are endocrine disruptors were reported to cause a decline in zebrafish sexual development. Furthermore, in the zebrafish testis tissue, it has been reported that pathological changes such as basement membrane separation, expansion in the sperm canal, interstitial changes, in the zebrafish ovarian tissue, increase in atretic oocytes, degenerative mineralization and interstitial proteinaceous fluid accumulation has been found. Molina et al. (2018) applied bisphenol A to female zebrafish in which follicles are affected, follicular populations are observed at different atresia levels, atretic degenerations and changes occur between the oocyte and follicular cells in primary follicles, hyalinization in oocyte cytoplasm in cortical alveolar oocytes, follicular atresia, follicular atresia, vacuolization in oocytes, degeneration of all components in mature oocytes, and structural deterioration in all organelles.
The findings of this study suggest that the use of fungicides which are frequently used in agriculture today should be controlled. In our study, mancozeb, a type of dithiocarbamate pesticide, has been found to cause deformations and adverse effects on the zebrafish (Danio rerio) ovary tissue. All these histological changes are closely related to reproduction. Great efforts are needed to understand the dithiocarbamate pesticide adverse effect on the reproductive system of animals and humans.
References
- Baligar PN, Kaliwal BB. 2001. Induction of gonadal toxicity to female rats after chronic exposure to mancozeb. Ind Health 39: 235–243. [PubMed] [Google Scholar]
- Bisson M, Hontela A. 2002. Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro. Toxicol Appl Pharmacol 180: 110–117. [CrossRef] [PubMed] [Google Scholar]
- Brouwer M, Huss A, van der Mark M, et al. 2017. Environmental exposure to pesticides and the risk of Parkinson's disease in the Netherlands. Environ Int 107: 100–110. [CrossRef] [PubMed] [Google Scholar]
- Chatterjee S, Dutta AB, Ghosh RAD. 1997. Impact of carbofuran in the oocyte maturation of catfish, Heteropneustes fossilis (Blosh). Arch Environ Contamin Toxicol 32: 426–430. [CrossRef] [PubMed] [Google Scholar]
- Cao F, Souders CL, Li P, et al. 2019. Developmental neurotoxicity of maneb: Notochord defects, mitochondrial dysfunction and hypoactivity in zebrafish (Danio rerio) embryos and larvae. Ecotoxicol Environ Saf 170: 227–237. [CrossRef] [PubMed] [Google Scholar]
- Dai YJ, Jia YF, Chen N, et al. 2014. Zebrafish as a model system to study toxicology. Environ Toxicol Chem 33: 11–17. [CrossRef] [PubMed] [Google Scholar]
- Di Giulio RT, Hinton DE. 2008. The Toxicology of Fishes. Taylor and Francis Group, Boca Raton: CRC Press, p. 1071 [Google Scholar]
- Dutta HM, Nath A, Adhikari S, Roy PK, Singh NK, Munshi JSD. 1994. Sublethal Malathion induced changes in the ovary of an air-breathing fish, Heteropneustes fossilis: a histological study. Hydrobiologia 294: 215–218. [CrossRef] [Google Scholar]
- Dutta HM, Dalal R. 2008. The effect of endosulfan on the ovary of bluegill sunfish: a histopathological study (Lepomis macrochirus). Int J Environ Res 2: 215–224. [Google Scholar]
- EPA. 2005. Prevention Pesticides and Toxic Substances, Reregistration Eligibility for maneb. EPA 7508C. [Google Scholar]
- Gullino ML, Tinivella F, Garibaldi A, Kemmitt GM, Bacci L, Sheppard B. 2010. Mancozeb: past, present, and future. Plant Disease 94: 1076–1087. [CrossRef] [PubMed] [Google Scholar]
- Haider S, Imbaraj R. 1988. In vitro effect of malathion and endosulfan on the LH-induced oocyte maturation in the common carp, Cyprinus carpio (L.). Water Air Soil Pollut 39: 27–31. [CrossRef] [Google Scholar]
- Hoo JY, Kumari Y, Shaikh MF, Hue SM, Goh BH. 2016. Zebrafish: a versatile animal model for fertility research. BioMed Res Int 2016: 9732780. [PubMed] [Google Scholar]
- Houeto P, Bindoula G, Hoffman JR. 1995. Ethylene bisdithiocarbamates and ethylenethiourea: possible human health hazards. Environ Health Perspect 103: 568–573. [CrossRef] [PubMed] [Google Scholar]
- Kackar R, Srivastava MK, Raizada BR. 1997. Studies on rat thyroid after oral administration of mancozeb: morphological and biochemical evaluations. J Appl Toxicol 17: 369–375. [CrossRef] [PubMed] [Google Scholar]
- Karakoç Ö, Nakiboğlu N. 2010. Dithiocarbamate pesticides and determination methods. BAÜ FBE Dergisi 12: 112–135. [Google Scholar]
- Kjeldsen LS, Ghisari M, Bonefeld-Jorgensen EC. 2013. Currently used pesticides and their mixtures affect the function of sex hormone receptors and aromatase enzyme activity. Toxicol Appl Pharmacol 272: 453–464. [CrossRef] [PubMed] [Google Scholar]
- Koc ND, Muslu MN, Kayhan FE, Colak SO. 2009. Histopathological changes in ovaries of zebrafish (Danio rerio) following administration of deltamethrin. Fresenius Environ Bull 18: 18721878. [Google Scholar]
- Kogan M, Lopez Greco LS, Romano LA, Rodriguez AM. 2000. Effects of cadmium on somatic and gonadal growth of juvenile females of the estuarine crab Chasmagnathus granulata (Brachyure: Grapsidae). Zool Stud 39: 344350. [Google Scholar]
- Luzio A, Monteiro SM, Roch E, Fontaínhas-Fernandes AA, Coimbra AM. 2016. Development and recovery of histopathological alterations in the gonads of zebrafish (Danio rerio) after single and combined exposure to endocrine disruptors (17-ethinylestradiol and fadrozole). Aquat Toxicol 175: 90–105. [CrossRef] [PubMed] [Google Scholar]
- Maqbool A, Ahmed I. 2013. Effects of Pesticide Monocrotophos (Organophosphate), on the Gonadal Development of Female Freshwater Murrel, Channa punctatus (Bloch). Int J Recent Sci Res 4: 1454–1458. [Google Scholar]
- Magar RS, Bias UE. 2013. Histopathological impact of malathion on the ovary of the freshwater fish Channa punctatus. Int Res J Environ Sci 2: 59–61. [Google Scholar]
- Mahadevaswami MP, Jadaramkunti UC, Hiremath MB, Kaliwal BB. 2000. Effect of mancozeb on ovarian compensatory hypertrophy and biochemical constituents in hemi-castrated albino rats. Reprod Toxicol 14: 127–134. [CrossRef] [PubMed] [Google Scholar]
- Maltby L, Brock TC, Van den Brink PJ. 2009. Fungicide risk assessment for aquatic ecosystems: importance of interspecific variation, toxic mode of action, and exposure regime. Environ Sci Technol 43: 7556–7563. [CrossRef] [PubMed] [Google Scholar]
- Molina AM, Abril N, Morales-Prietob N, et al. 2018. Evaluation of toxicological endpoints in female zebrafish after bisphenol A exposure. Food Chem Toxicol 112: 19–25. [CrossRef] [PubMed] [Google Scholar]
- Pandey AC. 1988. Impact of Endosulfan (Thiodan EC 35) on behavior and dynamics of oocyte development in the teleostean fish Colisa fasciatus. Ecotoxic Environ Saf 15: 221225. [CrossRef] [Google Scholar]
- Paro R, Tiboni GM, Buccione R, et al. 2012. The fungicide mancozeb induces toxic effects on mammalian granulosa cells. Toxicol Appl Pharmacol 260: 155–161. [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Pavlovic V, Cekici S, Kamenov B, Cırıcı M, Krtinic D. 2015. The effect of ascorbic acid on Mancozeb-induced toxicity in rat thymocytes. Folia Biol 61: 116–123. [Google Scholar]
- Pimentel D, Berger B, Filiberto D, et al. 2004. Water resources: agricultural and environmental issues. BioScience 54: 909–918. [CrossRef] [Google Scholar]
- PPDB. 2018. Pesticide Properties DataBase for Maneb. Retrieved from 2021-06-14. (http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/426.htm) [Google Scholar]
- Ram RN, Singh IJ, Singh DV. 2001. Carbofuran induced impairment in the hypothalamo-neurohypophyseal gonadal complex in the teleost, Channa puntatus (Bloch). J Environ Biol 22: 193–200. [PubMed] [Google Scholar]
- Rastogi A, Kulshrestha SK. 1990. Effect of sublethal doses of three pesticides on the ovary of a carp minnow Rasbora daniconius. Bull Environ Contam Toxicol 45: 742774. [CrossRef] [PubMed] [Google Scholar]
- Runkle J, Flocks J, Economos J, Dunlop AL. 2017. A systematic review of mancozeb as a reproductive and developmental hazard. Environ Int 99: 29–42. [CrossRef] [PubMed] [Google Scholar]
- Saha NC, Giri SK, Chatterjee N, Biswas SJ, Bej S. 2016. Acute toxic effects of mancozeb to fish Oreochromis mossambicus (WKH Peters, 1852) and their behavior. Int J Adv Res Biol Sci 3: 40–44. [Google Scholar]
- Saksena DN. 1999. Ichthyology: Recent Research Advances Science Publishers, p. 453. [Google Scholar]
- Sharma MR, Mushtaq R, Allayie SA, Vardhan H. 2016. Assessment of lethal toxicity of mancozeb and its consequences on the behavior of freshwater fish, Puntius ticto. J Int Acad Res Multidiscipl 4: 132–138. [Google Scholar]
- Srivastava P, Singh A. 2013. In vivo study of effects of dithiocarbamates fungicide (Mancozeb) and its metabolite ethylenethiourea (ETU) on fresh water fish Clarius batrachus. J Biol Earth Sci 3: 228–235. [Google Scholar]
- Tanguay RL. 2018. The rise of zebrafish as a model for toxicology. Toxicolog Sci 163: 3–4. [CrossRef] [PubMed] [Google Scholar]
- Trivedi N, Kackar R, Srivastava MK, Mithal A, Raizada RB. 1993. Effect of oral administration of fungicide mancozeb on thyroid gland of rat. Indian J Exp Biol 31: 564–566. [PubMed] [Google Scholar]
Cite this article as: Yön Ertuğ ND, Uzun E, Dinç T, Akbulut C. 2021. Effects of ethylene-bis-dithiocarbamate (Mancozeb) on zebrafish (Danio rerio) oocytes. Ann. Limnol. - Int. J. Lim. 57: 22
All Figures
 |
Fig. 1 Ovarian tissue of control group. PO: primary oocyte, CAO: cortical alveolar stage oocyte, VO: vitellogenic oocyte, M: mature oocyte, O: ooplasm, Kp: karyoplasm, ZR: zona radiata, A, B: H&E staining. |
| In the text | |
 |
Fig. 2 Ovarian tissue of 5 mg L−1 mancozeb exposed group, PO: primary oocyte, CAO: cortical alveolar stage oocyte, M: mature oocyte, AT: atretic oocyte, O: ooplasm, Kp: karyoplasm, ZR: zona radiata, FE: follicular epithelium, asteriks: detachment at epithelium, arrow: shrinkage at karyoplasm, A,D: Masson Trichrome, B: PAS, C: H&E staining. |
| In the text | |
 |
Fig. 3 Ovarian tissue of 7.5 mg L−1 mancozeb exposed group, CAO: cortical alveolar stage oocyte, VO: vitellogenic oocyte, M: mature oocyte, Ct: connective tissue, N: nucleus, Kp: karyoplasm, ZR: zona radiata, Fe: follicular epithelium, arrow: shrinking karyoplasm, square: vacuolization at cortical alveoli, yellow rectangle: deteriorated and fragmented in the zona radiata, red rectangle: separation between vitelline envelope and zona radiata, A,B: PAS, C: H&E staining. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


