| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 21 | |
| Number of page(s) | 8 | |
| DOI | https://doi.org/10.1051/limn/2021019 | |
| Published online | 21 October 2021 | |
Research Article
Age, growth, and mortality of silver carp Hypophthalmichthys molitrix (Valenciennes, 1844) in the middle and lower reaches of the Pearl River, and implications for management and conservation
1
Pearl River Fisheries Research Institute, Chinese Academy of Fishery Science, Guangzhou 510380, PR China
2
Guangzhou Scientific Observing and Experimental Station of National Fisheries Resources and Environment, Guangzhou 510380, PR China
3
Scientific Observing and Experimental Station of Fishery Resources and Environment in the Middle and Lower Reaches of Pearl River, Ministry of Agriculture and Rural Affairs, Guangzhou 510380, PR China
4
Key Laboratory of Aquatic Animal Immune Technology of Guangdong Province, Guangzhou, China, Guangzhou 510380, PR China
* Corresponding author: lijie1561@163.com
Received:
20
May
2021
Accepted:
26
September
2021
This study aimed to determine the age, growth, mortality, and population structure of the economically important cyprinid silver carp Hypophthalmichthys molitrix (Valenciennes, 1844) in the middle and lower reaches of the Pearl River. A total of 297 silver carp were sampled quarterly from the catches of gillnet fishermen, at six sites, between June 2019 and September 2020. Standard length of the specimens ranged from 130 to 585 mm, and body weight ranged from 45.5 to 3930 g. The length–weight relationship parameter b values reached 3.015. Age was determined through examination and measurements of fish scales, and the age composition of the sampled silver carp varied from 0+ to 4+. Fitting the new data to the von Bertalanffy growth model, we obtained an asymptotic size (L∞) of 1107 mm, k of 0.135, and t0 of −0.666 for silver carp in this stretch of the river. The calculated growth performance index ϕ and estimated longevity tmax were 5.22 and 21.56, respectively. The rates of total mortality, natural mortality, and fishing mortality were calculated as 0.4997, 0.1621, and 0.3377, respectively, while the exploitation ratio was evaluated as 0.6757. The overall results confirm overexploitation of this resource in the middle and lower reaches of the Pearl River. It was concluded that this species should be protected from capture until at least 790 mm in standard length, representing an optimal minimum size for capture to benefit conservation of the species and to sustainably develop this valuable fishery.
Key words: Silver carp / length–weight / von Bertalanffy growth function / capture standard / conservation
© EDP Sciences, 2021
1 Introduction
Freshwater fishes are largely overexploited worldwide, and many of the ecosystems that sustain them have become degraded (Dudgeon et al., 2006). China's large population and thriving economy have correspondingly increased the demand for fish as food, leading to situations of severe overexploitation (Guo et al., 2019; Chen et al., 2020). Historically, poorly regulated fish harvesting, in conjunction with other anthropogenic pressures, has resulted in dramatic declines in fishery resources, total catches, and biodiversity (Chen et al., 2020). To address the sustainable development of freshwater fisheries, it is necessary to establish effective fisheries management. Depressed fishery resources and the declining conservation status (and even extinction) of some endemic fish species have led to instances of fishing bans in the Yangtze River and other river basins in China. The recent fishing bans are unprecedented in their duration and extent (Chen et al., 2020). Determinations of fish growth parameters provide important and reliable scientific information to carry out regional assessments and conservation actions (de Santana et al., 2020). Basic data from studies of growth and population dynamics are input into various models used to evaluate and manage exploited fish stocks (Ju et al., 2016; Mehanna et al., 2018).
The silver carp Hypophthalmichthys molitrix (Valenciennes, 1844) is a freshwater cyprinid native to East Asia, and it has been introduced worldwide for aquaculture and controlling algal blooms (Froese and Pauly, 2020). As one of the most economically important fishes in China, its resources have been in a state of continuous decline since the 1980s as a result of water conservancy construction, water pollution, overfishing, and other factors (Liu et al., 2005; Tan et al., 2010; Chen et al., 2020). Conservation programs involving the silver carp, such as artificial fish propagation and release projects, have been implemented since 2006 (Chen et al., 2009). The population structure and growth characteristics of silver carp have been studied in the Yangtze River, Songhua River, and Minjiang River (Xiong et al., 2013; Pan et al., 2019; Wang, 2020; Wang et al., 2020). Studies of the biology and ecology of silver carp in the Pearl River have been undertaken since the 1980s (Lu, 1990), but few investigations have focused on decadal changes in its age, growth, and mortality.
The Pearl River is the longest river in southern China, exceeding 2000 km and stretching between 21°31––26°49h– N and 102°14––115°53– E (Editorial Committee of Encyclopedia of Rivers and Lakes in China, 2013); it is located in tropical and subtropical climate zones having an annual mean temperature range of 14–22 °C and long-term annual average precipitation of 1525.1 mm (Deng et al., 2018). Biological information on silver carp in the Pearl River would provide basic data that are needed for management of a sustainable fishery. Thus, this study aimed to (i) provide basic information on age, growth, mortality, and population structure of the silver carp, and (ii) propose reasonable management and protection measures for this species in the middle and lower reaches of the Pearl River.
2 Materials and methods
2.1 Fish sampling
This study was conducted in the middle and lower reaches of the Pearl River and included sites at Qianjiang, Xunjiang, Xijiang, and the Pearl River estuary. This extensive section of the Pearl River is an important fishing area for silver carp. Qianjiang and Xunjiang are main spawning grounds of silver carp; Xijiang and the Pearl River estuary are feeding ground and migration route. To determine current demographic parameters of silver carp, samples were collected quarterly, from June 2019 to September 2020, at six sites: Guiping, Tengxian, Wuzhou, Deqing, Zhaoqing, and Jiujiang (Fig. 1). Fish were obtained from local fishermen using gillnets (height 2 m × stretched length 100 m, 5–10-cm mesh). Each specimen collected was measured for standard length SL (to the nearest 1 mm) and body weight W (to the nearest 0.1 g), and 6–10 scales were removed from the region of the fish between the dorsal-fin origin and the lateral-line scales. The scales were then soaked in 4% sodium hydroxide solution and cleaned for investigation.
 |
Fig. 1 Map showing the six sampling sites in the middle and lower reaches of the Pearl River. |
2.2 Age determination
To determine the age of the sampled silver carp, the collected scales were examined and measured under a stereomicroscope (Olympus SZX16). The total radius of each scale (R: distance from the focus to the nth annulus) was measured to the nearest 0.01 mm. The scale edge was also measured for backcalculating the fish length at the time of the annulus formation. The sampled silver carp were classified into age groups based on the number of completed annuli (Fig. 2).
The relationship of fish body length to scale radius was based on the assumption that scale growth is proportional to fish growth, calculated as: L = a + b × R, where L is fish length (mm); R is scale radius (mm); b is slope value; and a is the initial fish length before scale formation (Lee, 1920; El-Nasr, 2017). Backcalculated fish length at the end of each year was calculated as: Ln = (L − a) × (Rn/R) + a, where Ln is the length at n years; L is the standard length at capture (mm); Rn is the scale radius at annulus n; R is total radius of the scale at the time of capture; and a is the intercept at y-axis (Lee, 1920; El-Nasr, 2017). To guarantee the independence of the data, only the last measurement was used for each individual.
 |
Fig. 2 Scale and annuli characteristics of silver carp sampled from the Pearl River. |
2.3 Estimation of the growth parameters
The relationship between SL (mm) and W (g) for the silver carp specimens was expressed by the equation W = α × Lb, where a is a coefficient related to body form, and b is an exponent that indicates isometric growth when b = 3 (Lleonart et al., 2000; Froese, 2006). Student's t-tests were used to test significance of the difference between b and the value 3, implemented with the function ‘hoCoef’ in the package FAS in R software.
Absolute growth rate was calculated as: (L2 − L1)/(t2 − t1) or (W2 − W1)/(t2 − t1); relative growth rate as: (L2 − L1)/L1 × (t2 − t1) or (W2 − W1)/W1 × (t2 − t1); instantaneous growth rate as: (lnL2 − lnL1)/(t2 − t1) or (lnW2 − lnW1)/(t2 − t1); and the index of growth as: Ce = (log10L2 − log10L1)/0.4343 × (t2 − t1) × L1, where W2, W1 and L2, L1 are the weights and lengths at ages t2 and t1 (Chen and Liu, 2017).
The von Bertalanffy growth function (VBGF) was used to determine the growth parameters as follows: Lt = L∞ × (1 − e−k (t−t0)), 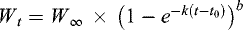 , where Lt and Wt are the SL (mm) and W (g) at age t (year); L∞ and W∞ are the mean asymptotic SL and W; k is the body growth coefficient (year−1); t is age; and t0 is the theoretical age when the specimen's size was zero (Bertalanffy, 1938; El-Nasr, 2017). The inflection age of body growth was calculated as: ti = ln b/k + t0. The growth performance index was calculated using the formula: ϕ = log10k + 2 × log10L∞ (Pauly and Munro, 1984). Longevity (tmax) was estimated using the growth parameters k and t0 as follows: tmax = 3/k + t0 (Pauly, 1983).
, where Lt and Wt are the SL (mm) and W (g) at age t (year); L∞ and W∞ are the mean asymptotic SL and W; k is the body growth coefficient (year−1); t is age; and t0 is the theoretical age when the specimen's size was zero (Bertalanffy, 1938; El-Nasr, 2017). The inflection age of body growth was calculated as: ti = ln b/k + t0. The growth performance index was calculated using the formula: ϕ = log10k + 2 × log10L∞ (Pauly and Munro, 1984). Longevity (tmax) was estimated using the growth parameters k and t0 as follows: tmax = 3/k + t0 (Pauly, 1983).
2.4 Mortality and exploitation rate
Following the Beverton–Holt (B-H) model (Beverton and Holt, 1956), total mortality (Z) was estimated based on coefficients related to average body lengths, thus: Z = k ×(l∞ − l)/(l − l–), where l– is minimum fishery landing SL, and l is mean fishery landing SL. Natural mortality (M) was estimated using an empirical equation (Pauly, 1980): lnM = − 0.0152 − 0.279 × ln L∞ + 0.6543 × ln k + 0.463 × ln T, where mean water temperature (T) is 23.48 °C. Fishing mortality (F) was calculated as F = Z − M. The exploitation ratio (E) was calculated as E = F/Z (Temple et al., 2020).
2.5 Critical age
The critical age (Tc, the optimum capture age) was determined using the equation of Yuan (1989): Tc = (K × t0 − ln M + ln (3 × K + M))/K. The critical standard length (Lc, the optimum capture length) and the critical body weight (Wc, the optimum capture body weight) were calculated using the value of Tc and the von Bertalanffy equation.
2.6 Minimum capture standard
Minimum capture length (LR) was estimated using the equation relating LR to E and the mean fishery landing l, thus: LR = l × E1/b (Xu and Zhang, 1988; Ju et al., 2016). Minimum capture body weight (WR) was estimated using the equation relating WR to E and the mean fishery landing weight W, thus: WR = E × W (Radway, 1953).
3 Results
3.1 Size and age structure of the silver carp
A total of 297 silver carp specimens were collected. The length range was 130–585 mm SL, with an average of 342.85 ± 94.95 mm SL, and dominant range of 248.0–437.9 mm SL. Body weight ranged from 45.5 to 3,930.0 g, with an average of 901.98 ± 673.61 g, and dominant range of 228.38–1,575.59 g (Fig. 3). The scales collected from 44 of the sampled silver carp were damaged, and therefore their age could not be determined; hence, 253 sampled fish were age-identified. The age range of the specimens was from 0+ to 4+ years. Among them, 41 fish (16.21%) were age 0+; 87 (34.39%) were age 1+; 84 (33.20%) were age 2+; 36 (14.23%) were age 3+; and 5 (1.98%) were age 4+ (Tab. 1). Fish in age groups 1+ and 2+ predominated. All the age 4+ silver carp were collected at Wuzhou.
 |
Fig. 3 Distributions of length frequency and weight frequency for the sampled silver carp. |
Age structure of the silver carp populations.
3.2 Length–weight relationship
In this study, the standard length and body weight relationship of the silver carp is given as a power function expressed by the equation W = 1.66 × 10−5 × L3.015 (Fig. 4). The results showed no significant difference between b = 3.015 and the value of 3 (p = 0.49), indicating that the growth rate of the silver carp was nearly isometric.
 |
Fig. 4 Relationship between standard length and body weight in the sampled silver carp. |
3.3 Growth
There is high correlation between scale radius and standard length in silver carp. The computed relationship between standard length and scale radius was expressed as L = 140.87 × R + 13.7. Table 2 lists the backcalculated lengths and weights at different years of life for the sampled silver carp.
The von Bertalanffy growth model yielded the following parameters for the silver carp: L∞ = 1107 mm, k = 0.135, W∞ = 25,015.73 g, and t0 = –0.666. The VBGF Lt is a non-inflection point curve that increases with age and then tends toward an asymptotic value (Fig. 5). The VBGF Wt is an S-type curve and has points of inflection, signifying that the body weight increases with age following a low–fast–low pattern, and then tends toward asymptotic (Fig. 5). Accordingly, the computed growth performance index (ϕ) was 5.22 for the silver carp. The age of growth inflection (ti) was 7.51, and estimated longevity (tmax) was 21.56.
Back-calculated and growth measurement of standard length and body weight of the silver carp.
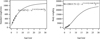 |
Fig. 5 Growth curves of standard length and body weight for silver carp from the Pearl River. |
3.4 Mortality
We determined Z = 0.4997 (evaluated with the B-H model), M = 0.1621, F = 0.3377, and E = 0.6757 for the sampled silver carp.
3.5 Capture standard
Optimum capture length Lc was calculated as 790.56 mm, and optimum capture body weight Wc was 9, 065.29 g. Minimum capture length LR and minimum capture body weight WR were 296.64 mm SL and 609.47 g, respectively.
4 Discussion
The present study estimates the age, growth, mortality, and population structure of silver carp in the middle and lower reaches of the Pearl River. The von Bertalanffy growth parameters L∞, k and t0 are basic data that are needed for input into the various models used for managing and assessing the status of exploited fish stocks; moreover, these parameters facilitate comparisons between the growth rates of different species or within the same species at different times or in different localities (Mehanna et al., 2018). Table 3 presents the growth parameters obtained for silver carp in this study alongside values presented by other authors. Parameters k and L∞ are interdependent (Gallucci and Quinn, 1979). In general, in this study the larger the value of k, then the smaller the value of L∞. The asymptotic length (L∞) of 1107 mm calculated here for silver carp in the Pearl River is relatively high compared with determinations for this species in other areas, such as in the upper Yangtze River (1037 mm), middle Yangtze River (1047 mm), Minjiang River (1067 mm), and Songhua River (746 mm), whereas the value of k that was determined is relatively lower than that for silver carp in the other locations. These results indicate that the silver carp populations in the middle and lower reaches of the Pearl River grow slower as compared with fish in other locations in China. The difference in growth parameters for silver carp in different drainages might be caused by environmental and ecological factors in different regions, including differences in population density and water temperatures (Ju et al., 2016).
In contrast, the values of parameter b remained within the expected range of 2.5–3.5 (Froese, 2006). The b value for silver carp in the middle and lower reaches of the Pearl River was 3.015, signifying nearly isometric growth. This value was close to that obtained for this species in the Upper Yangtze River (Xiong et al., 2013), Minjiang River (Wang, 2020), and Songhua River (Wang et al., 2020), but higher than that in the middle section of the Yangtze River (Pan et al., 2019). Differences in b values for the same species might be attributable to several factors, including differences in environmental conditions, growth phase, stomach fullness, sampling size, and length range (Froese et al., 2011).
Mortality coefficients for silver carp in the middle and lower reaches of the Pearl River are reported here for the first time. Determination of the natural mortality rate (M) is an important life-history parameter estimate for managing exploited fish populations (Gray et al., 2017; Sippel et al., 2017). Furthermore, M correlates with several other parameters, such as F, E, Tc and LR. Together and individually, these parameters can greatly influence stock assessment outcomes and subsequent management decisions (Gray et al., 2017; Sippel et al., 2017; Newman et al., 2000). In the current study, the estimates of F and E were greater than M, indicating that silver carp populations in this section of the Pearl River are heavily fished. Moreover, the exploitation rate (E) exceeded the value denoting optimal exploitation at E = 0.5 (Patterson, 1992; Gray et al., 2017); this further reveals that these stocks have been overfished.
The maximum age (tmax) of a fish species will be affected by the environmental conditions (Mehanna et al., 2018). In this study, the estimated longevity for silver carp was 21.56 years, whereas the previously reported maximum age of silver carp was 20 years in Florida, USA (Froese and Pauly, 2020). Age at sexual maturity of silver carp was generally 3 years in the Pearl River (Lu, 1990). In the present study, the age composition of the sampled silver carp varied from 0+ to 4+, with fish aged 1+ (34.39%) and 2+ (33.20%) dominant. Moreover, the younger age groups were dominant across the sampling period, indicating the risk of growth overfishing. Juvenile fish are a driving force of recruitment, allowing fish stocks to endure and recover (Ju et al., 2016). For instance, a break in the chain of recruitment will lead to collapse of a fishery if masses of juveniles are captured. Therefore, based on the data obtained here, it is recommended that the minimum capture size of silver carp in the Pearl River should be 790 mm SL.
The middle and lower reaches of the Pearl River are important habitats and fishing areas for silver carp, and all the samples in this study were collected in this section. However, because silver carp are widely distributed throughout the Pearl River, this study cannot reflect the situation of the stock in the whole basin; therefore, the age, growth, and mortality of silver carp in other regions of the Pearl River must likewise be studied.
Growth parameters of the silver carp from different localities.
5 Conclusion
This study of the age structure, von Bertalanffy growth parameters, mortality coefficients, and exploitation ratio of silver carp in the middle and lower reaches of the Pearl River provides vital data for managing the stock dynamics. To conserve this species and sustainably develop the fishery, in this stretch of the Pearl River, silver carp should be protected from capture until at least 790 mm in standard length.
Authors contributions
Zhu Shuli, Li Jie, and Li Xinhui designed the research and wrote the initial manuscript. Zhu Shuli, Wu Zhi, Zhang Yingqiu, Chen Weitao, and He Yujie performed the sampling and laboratory analyses.
Statement of conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The research was supported financially by the National Key R&D Program of China (2018YFD0900902), Pearl River fishery resources investigation and evaluation innovation team project (2020TD10, 2020ZJTD-04). The current study was carried out under the Laboratory Animal Management Principles of China. Cynthia Kulongowski with Liwen Bianji (Edanz) (www.liwenbianji.cn) edited the language of a draft of this manuscript.
References
- von Bertalanffy L. 1938. A quantitative theory of organic growth (inquiries on growth laws. II.). Human Biol 10: 181–213. [Google Scholar]
- Beverton RJH, Holt SJ. 1956. A review of methods for estimating mortality rates in fish populations, with special reference to sources of bias in catch sampling. Rapports et Proces-verbaux des Reunions. Conseil International Pour L'Exploration de la Mer. [Google Scholar]
- Chen DQ, Xiong F, Wang K, Chang YH. 2009. Status of research on Yangtze fish biology and fisheries. Environ Biol Fishes 85: 337–357. [CrossRef] [Google Scholar]
- Chen XJ, Liu BL. 2017. Fishery resources biology. Beijing, China: Science Press. [Google Scholar]
- Chen YS, Qu X, Xiong FY, Lu Y, Wang LZ, Robert MH. 2020. Challenges to saving China's freshwater biodiversity: Fishery exploitation and landscape pressures. AMBIO-A J Human Environ 49: 926–938. [CrossRef] [PubMed] [Google Scholar]
- Deng SL, Chen T, Yang N, Qu L, Li M, Chen D. 2018. Spatial and temporal distribution of rainfall and drought characteristics across the Pearl River basin. Sci Total Environ 619–620: 28–41. [CrossRef] [PubMed] [Google Scholar]
- de Santana HS, Tos CD, Minte-Vera CV. 2020. A review on the age and growth studies of freshwater fish in South America. Fish Res doi: 10.1016/j.fishres.2019.105410. [Google Scholar]
- Dudgeon D, Arthington AH, Gessner MO, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81: 163–182. [CrossRef] [PubMed] [Google Scholar]
- Editorial Committee of Encyclopedia of Rivers and Lakes in China. 2013. Encyclopedia of rivers and lakes in China, section of Zhujiang River Basin. Beijing, China: China Water and Power Press. [Google Scholar]
- El-Nasr TMAA. 2017. Age and growth of the fish, Gerres filamentosus (Cuvier, 1829) from Hurghada Red Sea, Egypt(Article). Egyptian J Aquat Res 43: 219–227. [CrossRef] [Google Scholar]
- Froese R. 2006. Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22: 241–253. [Google Scholar]
- Froese R, Tsikliras AC, Stergiou KI. 2011. Editorial note on weight-length relations of fishes. Acta Ichthyolog Piscator 41: 261–263. [CrossRef] [Google Scholar]
- Froese R, Pauly D. 2020. FishBase. World Wide Web electronic publication. Retrieved from www.fishbase.org. version (12/2020). [Google Scholar]
- Gallucci VF, Quinn TJ. 1979. Reparameterizing, fitting, and testing a simple growth-model. Trans Am Fish Soc 108: 14–25. [CrossRef] [Google Scholar]
- Gray CA, Barnes LM, Robbins WD, van der Meulen DE, Ochwada-Doyle FA, Kendall BW. 2017. Length- and age-based demographics of exploited populations of stout whiting, Sillago robusta Stead, 1908. J Appl Ichthyol 33: 1073–1082. [CrossRef] [Google Scholar]
- Guo CB, Chen YS, Rodolphe EG, et al. 2019. Biogeographic freshwater fish pattern legacy revealed despite rapid socio-economic changes in China. Fish Fisher 20: 857–869. [CrossRef] [Google Scholar]
- Hayer C-A, Breeggemann JJ, Klumb RA, Graeb BDS, Bertrand KN. 2014. Population characteristics of bighead and silver carp on the northwestern front of their North American invasion. Aquat Invas 9: 289–303. [CrossRef] [Google Scholar]
- Ju PL, Yang L, Lu ZB, et al. 2016. Age, growth, mortality and population structure of silver croaker Pennahia argentata (Houttuyn, 1782) and red bigeye Priacanthus macracanthus Cuvier, 1829 in the north-central Taiwan Strait. J Appl Ichthyol 32: 652–660. [CrossRef] [Google Scholar]
- Lee RM. 1920. A review of the methods of age and growth determination in fishes by means of scales. Ministry of Agriculture and Fisheries. Fishery Investigations, Series II. [Google Scholar]
- Liu SP, Duan XB, Chen DQ, Liao FC, Chen WJ. 2005. Studies on status of fishery resources in the middle reach of the yangtze river. Acta Hydrobiolog Sin 29: 708–711. [Google Scholar]
- Lleonart J, Salat J, Torres GJ. 2000. Removing allometric effects of body size in morphological analysis. J Theor Biol 205: 85–93. [CrossRef] [PubMed] [Google Scholar]
- Lu KX. 1990. Fishery Resources in the Pearl River Water System. Guangzhou, China: Guangdong Science and Technology Press. [Google Scholar]
- Mehanna SF, Osman AGM, Farrag MMS, Osman YAA. 2018. Age and growth of three common species of goatfish exploited by artisanal fishery in Hurghada fishing area, Egypt. J Appl Ichthyol 34: 917–921. [CrossRef] [Google Scholar]
- Newman SJ, Cappo M, Williams DM. 2000. Age, growth, mortality rates and corresponding yield estimates using otoliths of the tropical red snappers, Lutjanus erythropterus, L. malabaricus and L. sebae, from the central Great Barrier Reef. Fish Res 48: 1–14. [CrossRef] [Google Scholar]
- Pan WJ, Gao L, Yang H, et al. 2019. Studies on population structure and growth characteristics of Hypophthalmichthys molitrix in the Yichang to Jingzhou section of the middle branch of the Yangtze River. J Fishery Sci China 26: 362–370. [CrossRef] [Google Scholar]
- Patterson K. 1992. Fisheries for small pelagic species: an empirical approach to management targets. Rev Fish Biol Fish 2: 321–338. [CrossRef] [Google Scholar]
- Pauly D. 1980. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J Conseil Int pour l'Exploration de la Mer 39: 175–192. [Google Scholar]
- Pauly D. 1983. Length-converted catch curves: a powerful tool for fisheries research in the Tropics (part 1). Fishbyte 1: 9–13. [Google Scholar]
- Pauly D, Munro JL. 1984. Once more on the comparison of growth in fish and invertebrates. Fishbyte 2: 21. [Google Scholar]
- Radway Allen K. 1953. A method for computing the optimum size-limit for a fishery. Nature 172: 210. [Google Scholar]
- Sippel T, Lee HH, Piner K, Teo SLH. 2017. Searching for M: Is there more information about natural mortality in stock assessments than we realize?. Fisheries Res 192: 135–140. [CrossRef] [Google Scholar]
- Tan XC, Li XH, Lek S, et al. 2010. Annual dynamics of the abundance of fish larvae and its relationship with hydrological variation in the Pearl River. Environ Biol Fishes 88: 217–225. [CrossRef] [Google Scholar]
- Temple AJ, Stead SM, Jiddawi N, et al. 2020. Life-history, exploitation and extinction risk of the data-poor Baraka's whipray (Maculabatis ambigua) in small-scale tropical fisheries. J Fish Biol 97: 708–719. [CrossRef] [PubMed] [Google Scholar]
- Wang JL, Liu W, Li PL, Lu WQ, Tang FJ. 2020. Age and Growth Characteristics of Silver Carp (Hypophthalmichthys molitrix) in the Main Stream of Songhua River. Chin Agric Sci Bull 36: 140–146. [Google Scholar]
- Wang MY. 2020. Age and Growth of Hypophthalmichthys molitrix in the Middle of the Minjiang River. Chin Agric Sci Bull 36: 124–129. [Google Scholar]
- Williamson CJ, Garvey JE. 2005. Growth, fecundity, and diets of newly established silver carp in the middle mississippi river. Trans Am Fish Soc 134: 1423–1430. [CrossRef] [Google Scholar]
- Xiong F, Liu HY, Duan XB, Liu SP, Chen DQ. 2013. Age and growth of Hypophthalmichthys molitrix in Jiangjin of the Upper Reaches of the Yangtze River. J Southwest Univ (Natural Science Edition) 35: 28–35. [Google Scholar]
- Xu XC, Zhang QY. 1988. Age and growth of Saurida tumbil in the fishing ground of south-fujian and taiwan bank. J Oceanogr Taiwan Strait 7: 46–53. [Google Scholar]
- Yuan WW. 1989. Growth equations and critical age of some important commercial fishes in the northern South China Sea. South China Sea fisheries research (I). Guangzhou, China: Guangdong Technology Press. [Google Scholar]
Cite this article as: Zhu S, Wu Z, Zhang Y, Chen W, Li X, He Y, Li J. 2021. Age, growth, and mortality of silver carp Hypophthalmichthys molitrix (Valenciennes, 1844) in the middle and lower reaches of the Pearl River, and implications for management and conservation. Ann. Limnol. - Int. J. Lim. 57: 21
All Tables
Back-calculated and growth measurement of standard length and body weight of the silver carp.
All Figures
 |
Fig. 1 Map showing the six sampling sites in the middle and lower reaches of the Pearl River. |
| In the text | |
 |
Fig. 2 Scale and annuli characteristics of silver carp sampled from the Pearl River. |
| In the text | |
 |
Fig. 3 Distributions of length frequency and weight frequency for the sampled silver carp. |
| In the text | |
 |
Fig. 4 Relationship between standard length and body weight in the sampled silver carp. |
| In the text | |
 |
Fig. 5 Growth curves of standard length and body weight for silver carp from the Pearl River. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


