| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 20 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/limn/2021018 | |
| Published online | 12 October 2021 | |
Research Article
Phytoplankton dynamics in a seasonal stratified reservoir (Tillari), Western India
1
CSIR-National Institute of Oceanography, Dona Paula 403004, Goa, India
2
Environment and Life Sciences Research Center, Kuwait Institute for Scientific Research, Salmiya, 22017, Kuwait
3
Indian Institute of Technology, Kanpur 208016, India
* Corresponding author: naikayaz80@gmail.com
Received:
22
November
2020
Accepted:
19
September
2021
Phytoplankton are the primary producers in all the aquatic ecosystems and play an important role in key biogeochemical processes that are linked to the higher trophic levels and climate variability. The present study deals with the phytoplankton dynamics, biomass and physicochemical features in freshwater reservoir, Tillari, western India. The reservoir experience seasonal stratification and mixing associated changes in the biogeochemical aspects especially the phytoplankton community and chlorophyll a (hereafter, Chl a). The influence of seasonality was lesser in the deeper water in the reservoir. Buildup in phytoplankton biomass (up to 6.6 mg m−3) was observed in the upper strata of the water column (epilimnion) during the monsoon period (June–July) and winter (December) as a result of nutrient enrichment from the hypolimnion. Among nutrients, nitrate was associated with buildup of Chl a in the epilimnion during summer (r2 = 0.7). A total of 91 phytoplankton species were identified with major contribution by charophytes and chlorophytes. The dominant phytoplankton species belonged to genera Staurastrum, Cosmarium, Aulacoseira, Nephrocytium and Shroederia. Charophytes made a remarkable presence during the whole study period in the well oxygenated epilimnion as well as in the hypolimnion with relatively low oxygen. Diatom, the major silica sinking group was relatively less abundant. Keeping the importance of the reservoir in view, the understanding of phytoplankton community from this poorly explored reservoir with respect to influencing factors is a very vital baseline information. Thus, to design and evaluate the management strategies for the reservoir, continuous monitoring and processes studies is warranted.
Key words: Stratification / phytoplankton dynamics / reservoir / Western India
© EDP Sciences, 2021
1 Introduction
Reservoirs provide various ecosystem functions such as, source of drinking water for the local inhabitants, irrigation, flood control and power generation (Zhang et al., 2018; Huang et al., 2014). Any change in the state of reservoir could threaten biodiversity and thus cause substantial losses of ecosystem goods and services for human beings (Yang et al., 2017; Zhang et al., 2018). Eutrophication is one of the major concerns in the reservoirs in recent times causing algal blooms and thus have caused periodic deterioration of the water quality (Xiao et al., 2011; Wang et al., 2012; Ma et al., 2015). Therefore, adequate management is essential and of utmost importance in the reservoirs. A reservoir is an ideal ecosystem to study the physical, chemical and biological characteristics and their interactions. In tropical reservoirs, mixing and thermal stratification are the key processes influencing their water quality. Various studies have investigated the role of these processes in the phytoplankton community structure, very few have studied the dynamics of phytoplankton during these dominant processes. The dynamics of phytoplankton are traditionally considered to be influenced by abiotic variable like nutrient, light and temperature (Wichelen et al., 2016; Shi et al., 2020).
Phytoplankton, classically, characteristic association of microplankton, nanoplankton and picoplankton, are major contributor to primary production and play key role in aquatic food web, nutrient cycling and biogeochemical cycling (Isabwe et al., 2018; Shi et al., 2020). Each of the size classes has a characteristic association with the eutrophic and seasonal gradients (Watson et al., 1997). Distribution of nutrients in these systems is intrinsically linked with physical variables, with eutrophic water having relatively higher fraction of oxidized forms of nitrogen (N) and oligotrophic waters having a larger fraction of reduced N forms. However, when the physico-chemical condition of the system changes from one state to another, like from eutrophic to oligotrophic and vice versa, it is reflected with a change in phytoplankton biomass, abundance, and species composition. Such conditions occur seasonally in lakes and reservoirs where low nutrient surface water is progressively replaced with nutrient-rich water in space and time, entraining changes in phytoplankton biomass, composition and abundance. Such locations are ideal sites to study the control exercised by hydrography on phytoplankton. There is always complexity in studying the phytoplankton community due to the wide range of factors influencing it (Huszar, 1996; Dellamano-Oliveira et al., 2003). Studies show that the vertical dynamics of the phytoplankton is related to the water column-mixing regime (Xiao et al., 2011). The interaction of the climatological and hydrological factors and their influence over other environmental factors affect the biological distribution in the aquatic systems. Phytoplankton dynamics across seasons has been well investigated in aquatic ecology and various studies have described the patterns and underlying mechanisms of the seasonal dynamics (Sommer et al., 1986; Marshall and Peters, 1989; Vanni and Temte, 1990; Hansson et al., 1998; Rothhaupt, 2000; Ahmed and Wanganeo, 2015). The physical factors of the environment related to thermal stability of the water column are important in determining the structure of phytoplankton (Naselli-Flores et al., 2007).
Temperature and incident radiations favor rapid stratification in an aquatic ecosystem (Lewis, 1973) resulting in two separate layers of water, the epilimnion, and hypolimnion separated by the lacustrine interface metalimnion (Becker et al., 2009). Stratification has important implication for phytoplankton succession that frequently culminates in domination by a few species for long period of time (Soares et al., 2009). Thermally stratified reservoirs represent an ideal system to investigate phytoplankton community structure along with physical and chemical drivers (Lawson and Anderson, 2007). A corollary objective was thus to demonstrate how the physico-chemical factors influence the phytoplankton dynamics and biomass by establishing a fixed station at Tillari reservoir, western India.
2 Material and methods
2.1 Study area and sampling
The Tillari Reservoir (15.76° N, 74.09° E), constructed on the Tillari River, is located at the foothills of the Western Ghats, India (Fig. 1). The total storage capacity of the reservoir is about 0.45 billion m3 (Kurian et al., 2012; Narvenkar et al., 2013). The water level in the reservoir varies during the spring inter-monsoon (SIM, March to May) and during the southwest monsoon (SWM, June to September). The stored water is used for irrigation, generation of electricity, and domestic purpose. While one of the outlets is used for overflow, the outflow of the turbines was at 64-m reservoir level (Shenoy et al., 2021). During summer, when the temperature rises, the reservoir experience stratification, which causes oxygen depletion in the hypolimnion. The reservoir is dimictic, where, the water column is vertically mixed during northeast (winter) and southwest monsoon (Kurian et al., 2012).
Water sampling was carried out at a fixed station with maximum depth from March 2010 to August 2011. Water samples were collected using 5 L Niskin sampler, mounted on a polypropylene wire and fitted with reversing thermometer to measure temperature at different depth.
 |
Fig. 1 Map showing sampling location (Tillari reservoir). |
2.2 Environmental variables
Sampling for dissolved oxygen (DO) was done carefully using a tygon tubing fitted with a glass tube. The sampling tube was connected to the nozzle of the Niskin sampler, and the water was allowed to flow into the sample bottle filling from the bottom up. Care was taken to ensure no bubble was trapped in the sampling tube or the bottle. The samples were fixed with respective reagents after overflowing three times the sample bottle volume. It was fixed immediately upon collection by simultaneous additions of 1 mL each of Winkler A and Winkler B reagents. The sample was later analysed in the laboratory following the titration method as detailed in Grasshoff (1983).
Nutrient samples (nitrate, phosphate and silicate) were collected in 100-mL high density polyethylene bottles. The sample bottle was rinsed thoroughly before collection. After collection, the samples were stored in ice and later analysed in the laboratory, using the SKALAR autoanalyzer following Grasshoff (1983).
2.3 Phytoplankton biomass (Chl a)
For measurement of phytoplankton biomass (chlorophyll a), 1 liter water sample was collected from discrete depths using Niskin sampler and transferred to brown HDPE bottles. Samples were transported to laboratory in ice box. Known volume of water was filtered through 47 mm glass fiber filters (Whatman, 0.7 μm) and extracted in 90% acetone for 24 h at ‒20 °C in the dark. The extract was analyzed using Turner Designs, AU-10, fluorometer (UNESCO, 1994). Quantification and integration of Chl a was done following JGOFS protocol (Knap et al., 1996).
2.4 Phytoplankton analysis
A sub-sample of 250 ml collected from niskin sampler, fixed with 2% Lugol's iodine and stored in the dark at room temperature until analysis. A settling and siphoning procedure was followed to concentrate the samples. Concentrated samples were then examined microscopically in a Sedgewick-Rafter plankton counting chamber (Structure Probe, Inc., West Chester, PA, USA) under inverted microscope (Olympus IX 51;4009) at ×200 and ×400 magnification. A total of 2 mL of sample was analyzed in duplicate and the average values are presented here. Phytoplankton species were identified based on the morphology with the help of standard taxonomic literature (Randhawa, 1959; Presscot, 1962; Pal et al., 1962; Penak, 1978; Michael and Sharma, 1988; Edmondson, 1992). The species diversity (H), ranging from 0 to infinity was calculated according to Shannon and Wiener (1949) and Tian et al. (2013). Phytoplankton predominant indices (d) were calculated according to Berger and Parker, (1970):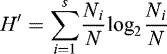
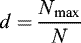
where Ni, Nmax, N and S are number of individuals of the ith species, number of individuals of abundant species, total number of individuals, and total species (Tian et al., 2013).
3 Results
3.1 Hydrographic conditions
Some of the hydrography conditions of the study area are detailed in studies by Kurian et al. (2012); Naqvi et al., 2018; Shenoy et al., 2021, however vertical profiles of representative season corresponding to stratification and vertical mixing period is presented here (Fig. 2). During the study the water column temperature varied between 24 °C and 32.9 °C. High temperature was observed during the summer (April to June), which resulted in stratification, thus leading to deoxygenation in the hypolimnion. During the summer, dissolved oxygen concentration with below 20 m depth, was below detection limit with the occurrence of hydrogen sulphide in the deeper depths. This condition changed with the onset of monsoon, which resulted in the mixing and re-oxygenation of the entire water column. The system was again weakly stratified during the warm October month, but anoxic or suboxic conditions were not observed. The winter observed between December and February again resulted in mixing of the entire water column, thus repleting the water column with nutrients (nitrate, phosphate and silicate). However, nitrate in the epilimnion was below detection limit during the summer.
 |
Fig. 2 Vertical profile of dissolved oxygen, temperature and chlorophyll a ( a: February2011 b: May, 2010 and c: August 2011) and nitrate, phosphate and silicate. |
3.2 Phytoplankton biomass (Chl a)
The vertical profiles of representative biogeochemical parameters and their variations with time is shown in Figures 2 and 3. Chlorophyll a concentration showed a marked variation on temporal scale in the reservoir. Vertically, the Chl a concentration was high in the epilimnion-metalimnion throughout the year, however, buildup in biomass was observed during the onset of warm period (March–April). The concentration slightly decreased during strong stratification (May–June). Maximum concentration (6.6 mg m−3) was observed in epi-metalimnetic layer during rainy period (south-west monsoon, July). The concentration was low in the hypolimnion during most part of the year, however, the mixing period (January) was represented with well mixed chlorophyll concentration (>2 mg m−3) throughout the water column (Fig. 2). Depth integrated Chl a (0‒50m) also showed a similar pattern of distribution with concentration varying between 19 and 85 mg m−2 with higher concentration observed during July 2011 (Fig. 3), when the water was enriched with nutrients.
 |
Fig. 3 Temporal variation in chlorophyll a:Dark filled bars represent integrated Chl a and dark line represents surface Chl a. |
3.3 Phytoplankton community composition
A detailed list of phytoplankton is presented in Table 1. A total of 91 phytoplankton species were identified with maximum taxa represented by green algae. The number of blue-green algae and diatoms made relatively less contribution to the total number. Temporal variation of total species number is presented in Table 2. The number of species varied between 37 (December) and 65 (June) with maximum species corresponding to charophytes throughout the study period. About 17 species were found to be dominant in this system with predominant indices varying between 0.001 and 0.34 (Tab. 3). Some of the dominant species included charophytes (Cosmarium contractum, Cosmarium depressum, Cosmarium sp., Staurastrum leptocladum, Staurastrum lunatum, Staurastrum manfeldtii, Staurastrum proboscideum and Staurastrum pseudopelagicum), chlorophytes (Closteriopsis acutum, Dictyosphaerium sp., Nephrocytium limneticum, Shroederia satigera, Sphaerocystis sp., Tetraedron regulare and Tetraedron trigonum), cyanobacteria (Aphanocapsa sp.), diatom (Aulacoseira granulata).
List of phytoplankton species in Tillari reservoir (March 2010–August 2011).
Temporal variation of phytoplankton species number in Tillari reservoir.
Variation of predominant species of phytoplankton in Tillari (Predominant indices).
3.4 Phytoplankton abundance and diversity
The phytoplankton abundance in the reservoir exhibited distinct temporal variation. The abundance varied between 0.3 to 161 × 104 cells L–l (Fig. 4). A decreasing trend in abundance was observed from March to May 2011 and February 2010 followed by an increase in maximum abundance during July 2011. The phytoplankton community showed vertically well mixed pattern during the winter convection (January), however, the abundance was generally high in the upper strata of the water column (epi-metalimnion).
The results showed that different taxonomic groups of phytoplankton reflect almost the similar pattern in distribution (Fig. 5). The cell abundance with respect to charophytes, chlorophytes, diatom and cyanobacteria varied up to 98.8, 40.8, 11.6 and 9.6 × 104 cells L–l indicating dominance of charophytes and chlorophytes. Maximum cell abundance (×104 L−1) of phytoplankton groups during stratification period varied up to 43.2 (charophytes) in the metalimnion and 5.9 (cyanobacteria) in the hypolimnion. During non-stratification period, the cell number of charophytes was as high as 98.8 × 104 L−1 in the epi-metalimnion and 7.5 × 104 cells L−1 in the hypolimnion (Fig. 6A and B). During the low oxygen conditions (April–June), cyanobacteria were higher in the hypolimnion, however, in the metalimnion the green algae, charophytes were abundant. Interestingly, the genera Staurastrum among the charophyte group was present in the water column throughout the study. Species of Staurastrum (Staurastrum leptocladum, and S. manfeldtii) and diatom Aulacosiera granulata were present in the low oxygen conditions during the stratification period. Diversity indices on phytoplankton cell abundance were calculated and ranged between 2.8 and 3.5 with and the minimum values during winter and higher during summer stratification period (Fig. 7). These values suggest that the phytoplankton species diversity was high during summer.
 |
Fig. 4 Vertical profile of phytoplankton cell abundance in Tillari reservoir. |
 |
Fig. 5 Vertical profile of different phytoplankton taxonomic groups. |
 |
Fig. 6 Vertical distribution of phytoplankton cells in different strata, A: during stratification period B: during mixing period, Chlor: chlorophytes, Char: charophytes, Cyan: cyanobacteria, Diat: diatom. |
 |
Fig. 7 Temporal variation in phytoplankton diversity index. |
4 Discussion
Tropical reservoirs experience physicochemical changes because of seasonal climatic conditions; however, such variations differ in amplitude and intensity. Phytoplankton succession and distribution in space and time have been reported to be governed by an interrelated effect of various physico-chemical conditions including light, temperature, oxygen, and nutrients (Smith, 1924; Marshall, 1965; Hutchinson, 1967; Sommer and Gliwicz, 1986; Yildiz et al., 2007; Davies et al., 2009; Wanganeo, 2010). Temperature is one of the major drivers influencing the phytoplankton through nutrient distribution in an aquatic system. The low temperature during the winter led to an increase in the water density, and convection resulting in complete mixing in the reservoir. High temperature resulted in water column stratification during summer. Stratification in lakes and reservoirs during summer results in a nutrient deficient epilimnion, chemocline in metalimnion and nutrient rich hypolimnion (Tyler and Vyverman, 1996; Gervais, 1998, 2003; Adler et al., 2000). Intense stratification inhibits the replenishment of nutrients and phytoplankton growth (Doyon et al., 2000). Seasonal thermal gradient was recorded in the Tillari reservoir with epilimnion ‒ hypolimnion temperature variation of 8 °C (Fig. 2) during stratification compared to ∼1 °C during mixing period. The observed changes in stratification in Tillari reservoir not only resulted in an establishment of contrasting but also differential availability of nutrients in the surface layers as reported earlier by Kurian et al. (2012). Surface water was nitrate deficient during the stratification; however, the concentration was relatively high (up to 4.8 μM) during the rainy season. The seasonal changes in physicochemical conditions in the system was well reflected in phytoplankton biomass (Chl a) and community structure. As a consequence, we observed maximum Chl a concentration (6.6 mg m−3) in epi-metalimnion during monsoon precipitation that decreased with increasing depth down in the water column. Subsequently, light limitation and water turbidity might have resulted in low biomass in the subsurface layers (hypolimnion), though nutrients were sufficient in the surface water. During winter, convective mixing to some extent resulted in homogeneous distribution in chlorophyll, however the concentration during this period was relatively low (Fig. 3). Nitrate was seen as an important parameter in surface water significantly (r2 = 0.7, n = 10) influencing the phytoplankton biomass (Fig. 8). It is well known that the nutrients are key factors influencing seasonal dynamics of phytoplankton (Lope et al., 2009; Ward et al., 2011). Water depth can also strongly affect the phytoplankton community (Leland, 2003). In the present study, phytoplankton seems to be controlled by the stratification. During summer, phytoplankton biomass was nitrate limited due to strong stratification compared to those during monsoon and winter convection.
Phytoplankton groups, charophytes and chlorophytes were abundant in the epi-metalimnion strata in Tillari reservoir (Tab. 3). This study indicates charophytes as a key component of phytoplankton community that reached the highest relative dominance outcompeting other groups and influence the diversity pattern. Charophytes are generally most common and diverse in oligotrophic lakes (Gerrath, 1993), however, highly sensitive to changes in the environmental condition and thus are considered as bioindicators of pristine conditions (Coesel, 1983, 2001). The distribution of charophytes in this study showed that the most diverse taxa corresponding to Staurastrum was dominant among all the phytoplankton. It is also widely accepted that Staurastrum is one of the most common genera found in lakes and reservoirs (Gerath, 1993). Interestingly, charophytes and cyanobacteria were also present in the low oxygen waters during the stratification period. Zhu et al. (2010) reported chlorophytes to be dominant at higher concentrations of NO−3 and PO3−4 whereas, cyanobacteria dominate in the waters with relatively low concentrations of these nutrients. Our data suggests co-existence of both groups in Tillari reservoir. The presence of phytoplankton in the low oxygen waters in the hypolimnion is generally attributed to their physiological stability and adaptability to such conditions. Such regions are physically stable during stratification and are avoided by the major grazers (Lass, 2000) and thus phytoplankton have less grazing pressure (Reynolds, 1992; Pedros-Alio et al., 1995; Tyler and Vyverman, 1996; Gervais, 1998; Camacho et al., 2000). Seasonal succession of phytoplankton can be greatly influenced by factors such as temperature, nutrients, and hydrology (Peng et al., 2012). Diatom prefer cooler environment (Silva et al., 2005) however, in the present study the surface water temperature was higher (>25 °C) and we observed the dominance of Aulacosiera granulata throughout the year. During persistent thermal stratification, Aulacosiera granulata tend to sink out of the euphotic zone, and thus was present in the lower depth. This species was homogeneously distributed in the water column during mixing periods.
Cyanobacteria proliferate at high temperature, high phosphate concentration, and water column stability (Smith, 1983; Paerl, 1988; Marinhos and Huszar, 2002; Islam et al., 2012). These conditions were observed in the Tillari reservoir during the summer. Cyanobacteria were present in Tillari reservoir during summer. High performance liquid chromatography (HPLC) based studies by Kurian et al. (2012) revealed presence of higher concentration of zeaxanthin (marker pigment of cyanobacteria) in the surface waters during stratification period. Presence of charophytes and chlorophytes during the stratification period and in the rainy season is in agreement with the results reported from other reservoirs (Ramirez and Diaz, 1994; Tucci and Santanna, 2003). The maximum phytoplankton density was observed during the rainy period and is related to nutrient availability during this period. Temporal variations in phytoplankton density in tropical lakes are normally controlled by the availability of nutrients and subsurface radiations (Eteves, 1998), which are in turn influenced by the external and internal conditions (wind, rain, stratification, and mixing). During winter mixing (January) and rainy period (July–August), the phytoplankton community was dominated by green algae, charophytes and cyanobacteria in the epi-metalimnion. However, these groups were also present in deeper water. The mixing events allow the phytoplankton to enter from deep into the euphotic zone (Margalef, 1983) and result in vertically heterogeneous distribution (Kullenberg, 1978). Kurian et al. (2012) also reported phytoplankton community dominated by cyanobacteria, green algae with minor presence of diatoms in the epilimnion as well as in deeper layer during winter mixing.
Rainfall has been reported to be potentially affecting phytoplankton growth in reservoirs worldwide (Robson and Hamilton, 2003; Behrenfeld, 2010). In the present study, charophytes and chlorophytes were dominant during the rainy period as well indicating that the mixing regime played a key role in nutrient availability and selection of certain phytoplankton groups. The study also yielded valuable and complementary information on understanding the phytoplankton dynamics in the reservoirs, their distribution and tolerance towards the environmental conditions. Though nutrients were present, they were not enough to support the phytoplankton biomass during some seasons. The presence of charophytes indicates the oligotrophic nature of the system however further detailed investigation is needed for better understanding and management of reservoir health.
 |
Fig. 8 Relationship between surface nitrate and chlorophyll concentration. |
5 Conclusion
The Tillari reservoir is seasonally stratified during summer with nutrient depleted condition in epilimnion and nutrient replete underlying hypolimnion. Seasonal rain and winter cooling resulted in the water column mixing and thus homogenous conditions which influence phytoplankton biomass and community structure. Chlorophyll a concentration was high when the precipitation occurred, however light limitation may have played a role restricting the buildup of biomass in subsurface layers as well as surface during post rainy period. Seasonal dynamics of phytoplankton in the reservoir was controlled by different factors such as temperature, light, nutrients etc. We observed seasonally diverse speciation with the majority of taxa corresponding to charophytes, Staurastrum (a major trophic indicator in the aquatic system) suggesting the system to be of oligotrophic state. This study provides the baseline data on phytoplankton from this reservoir however, further studies with horizontal transects and long term monitoring are warranted for better understanding and management of the health of the system.
Acknowledgements
The authors wish to thank the Director, CSIR-NIO and management of the Tillari reservoir for permitting us to carry out this study. We are grateful to our colleagues, Mr. H. Dalvi, Anand Methar and B.R Thorat for their assistance during sampling. This study was carried as part of the CSIR funded project “INDIAN IDEA” (PSC0108). This is NIO's contribution no 6807.
References
- Adler M, Gervais F, Siedel U. 2000. Phytoplankton species composition in the chemocline of mesotrophic lakes. Arch Hydrobiol 55: 513–530. [Google Scholar]
- Ahmed A, Wanganeo A. 2015. Phytoplankton succession in a tropical freshwater lake, Bhoj Wetland (Bhopal, India): spatial and temporal perspective. Environ Monit Assess 187: 1–12. [CrossRef] [PubMed] [Google Scholar]
- Becker V, Huszar VLM, Crossetti LO. 2009. Responses of phytoplankton functional groups to the mixing regime in a deep subtropical reservoir. Hydrobiologia 628: 137–151. [CrossRef] [Google Scholar]
- Behrenfeld MJ. 2010. Abandoning Sverdrup's Critical Depth Hypothesis on phytoplankton blooms. Ecology 91: 977–989. [PubMed] [Google Scholar]
- Berger WH, Parker FL. 1970. Diversity of planktonic foraminifera in deep-sea sediments. Science 168: 1345–1347. [CrossRef] [PubMed] [Google Scholar]
- Camacho A, Vicente E, Miracle MR. 2000. Ecology of a deep-living Oscillatoria (Planktothrix) population in the sulphide-rich waters of a Spanish karstic lake. Arch fur Hydrobiol 148: 333–355. [CrossRef] [Google Scholar]
- Coesel PFM. 1983. The significance of desmids as indicators of the trophic status of freshwaters. Schweizerische Zeitschrift fur Hydrol 45: 388–393. [Google Scholar]
- Coesel PFM. 2001. A method for quantifying conservation value in lentic freshwater habitats using desmids as indicator organisms. Biodivers Conserv 10: 177–187. [CrossRef] [Google Scholar]
- Davies OA, Abowei JF, Otene BB. 2009. Seasonal abundance and distribution of plankton of minichinda stream, Niger Delta, Nigeria. Am J Sci Res 2: 20–30. [Google Scholar]
- Dellamano-Oliveira MJ, Senna PA, Taniguchi GM. 2003. Limnological characteristics and seasonal changes in density and diversity of the phytoplanktonic community at the Caçó pond, Maranhão State, Brazil. Braz Arch Biol Technol 46: 641–651. [CrossRef] [Google Scholar]
- Doyon P, Klein B, Ingram RG, Legendre L, Tremblay JE, Therriault JC. 2000. Influence of wind mixing and upper-layer stratification on phytoplankton biomass in the Gulf of St. Lawrence. Deep. Res. Part II Top. Stud. Oceanography 47: 415–433. [Google Scholar]
- Gerrath JF. 1993. The biology of desmids: a decade of progress. Progr Phycol Res 9: 79–192. [Google Scholar]
- Gervais F. 1998. Ecology of cryptophytes coexisting near a freshwater chemocline. Freshw Biol 39: 61–78. [CrossRef] [Google Scholar]
- Gervais F. 2003. Small-scale vertical distribution of phytoplankton, nutrients and sulphide below the oxycline of a mesotrophic lake. J Plankton Res 25: 273–278. [CrossRef] [Google Scholar]
- Grasshoff P. 1983. Methods of seawater analysis. Verlag Chemie FRG 419: 61–72. [Google Scholar]
- Hansson LA, Annadotter H, Bergman E, et al. 1998. Minireview: Biomanipulation as an application of food-chain theory: constraints, synthesis, and recommendations for temperate lakes. Ecosystems 1: 558–574. [CrossRef] [Google Scholar]
- Huang T, Li X, Rijnaarts H, et al. 2014. Effects of storm runoff on the thermal regime and water quality of a deep, stratified reservoir in a temperate monsoon zone, in Northwest China. Sci Total Environ 485–486: 820–827. [PubMed] [Google Scholar]
- Huszar VLM. 1996. Planktonic algae, other than desmids of three Amazonian systems (Lake Batata, Lake Mussara and Trombetas River), Para, Brazil. Amazoniana 14: 37–73. [Google Scholar]
- Hutchinson EG. 1967. Introduction to lake biology and the limnoplankton. A Treatise Limnol 1115p. [Google Scholar]
- Isabwe A, Yang JR, Wang Y, Liu L, Chen H, Yang J. 2018. Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Sci Total Environ 630: 658–667. [PubMed] [Google Scholar]
- Islam MN, Kitazawa D, Park HD. 2012. Numerical modeling on toxin produced by predominant species of cyanobacteria within the ecosystem of Lake Kasumigaura, Japan. Proc Environ Sci 13: 166–193. [CrossRef] [Google Scholar]
- Knap AH, Michaels A, Close AR, Ducklow H, Dickson AG. 1996. Protocols for the joint global ocean flux study (JGOFS) core measurements. JGOFS, Reprint of the IOC Manuals and Guides No. 29, UNESCO 1994, 19. [Google Scholar]
- Kullenberg GEB. 1978. Vertical Processes and the Vertical-Horizontal Coupling, In: Spatial Pattern in Plankton Communities, Springer US, 43–71 p. [Google Scholar]
- Kurian S, Roy R, Repeta DJ, et al. 2012. Seasonal occurrence of anoxygenic photosynthesis in Tillari and Selaulim reservoirs, Western India. Biogeosciences 9: 2485–2495. [CrossRef] [Google Scholar]
- Lass S. 2000. How do migrating daphnids cope with fish predation risk in the epilimnion under anoxic conditions in the hypolimnion? J Plankton Res 22: 1411–1418. [CrossRef] [Google Scholar]
- Lawson R, Anderson MA. 2007. Stratification and mixing in Lake Elsinore, California: An assessment of axial flow pumps for improving water quality in a shallow eutrophic lake. Water Res 41: 4457–4467. [PubMed] [Google Scholar]
- Leland HV. 2003. The influence of water depth and flow regime on phytoplankton biomass and community structure in a shallow, lowland river. Hydrobiologia 506–509: 247–255. [CrossRef] [Google Scholar]
- Lewis WM. 1973. The thermal regime of Lake Lanao (Philippines) and its theoretical implications for tropical lakes1. Limnol Oceanogr 18: 200–217. [CrossRef] [Google Scholar]
- Lope M, Chan K-S, Ciannelli L, Reid PC, Stige LC, Stenseth NC. 2009. Effects of environmental conditions on the seasonal distribution of phytoplankton biomass in the North Sea. Limnol Oceanogr 54: 512–524. [CrossRef] [Google Scholar]
- Ma WX, Huang TL, Li X, Zhang HH, Ju T. 2015. Impact of short-term climate variation and hydrology change on thermal structure and water quality of a canyon-shaped, stratified reservoir. Environ Sci Pollut Res 22: 18372–18380. [CrossRef] [Google Scholar]
- Margalef R. 1983. Limnologia. Ediciones Omega, S.A., Barcelona. 1010 p. Limnol Oceanogr 29: 1349–1349. [Google Scholar]
- Marinho MM, Moraes Huszar VL De. 2002. Nutrient availability and physical conditions as controlling factors of phytoplankton composition and biomass in a tropical reservoir (Southeastern Brazil). Arch fur Hydrobiol 153: 443–468. [CrossRef] [Google Scholar]
- Marshall CT, Peters RH. 1989. General patterns in the seasonal development of chlorophyll a for temperate lakes. Limnol Oceanogr 34: 856–867. [CrossRef] [Google Scholar]
- Marshall HG. 1965. the Annual Distribution and Stratification of Phytoplankton at Aurora Lake, Portage. Ohio J Sci 65: 190–202. [Google Scholar]
- Michael RG, Sharma BK. 1988. Fauna of India and adjacent countries. Indian Cladocera (Crustacean: Branchiopoda: Cladocera). India: Zoological Survey of India. [Google Scholar]
- Naqvi SWA, Lam P, Narvenkar G, et al. 2018. Methane stimulates massive nitrogen loss from freshwater reservoirs in India. Nature Communications 9: 1–10. [CrossRef] [PubMed] [Google Scholar]
- Narvenkar G, Naqvi SWA, Kurian S, et al. 2013. Dissolved methane in Indian freshwater reservoirs. Environ Monit Assess 185: 6989–6999. [PubMed] [Google Scholar]
- Naselli-Flores L, Barone R, Chorus I, Kurmayer R. 2007. Toxic cyanobacterial blooms in reservoirs under a semiarid Mediterranean climate: the magnification of a problem. Environ Toxicol 22: 399–404. [PubMed] [Google Scholar]
- Paerl HW. 1988. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol Oceanogr 33: 823–843. [Google Scholar]
- Pal DP, Kundu BC, Sudarlingam PVS, Venkataraman GS. 1962. Charophyta. New Delhi: Indian council of agricultural research. [Google Scholar]
- Pedros-Alio C, Massana R, Latasa M, Garci-Cantizano J, Gasol JM. 1995. Predation by ciliates on a metalimnetic Cryptomonas population: feeding rates, impact and effects of vertical migration. J Plankton Res 17: 2131–2154. [CrossRef] [Google Scholar]
- Peng S, Qin X, Shi H, Zhou R, Dai M, Ding D. 2012. Distribution and controlling factors of phytoplankton assemblages in a semi-enclosed bay during spring and summer. Mar Pollut Bull 64: 941–948. [PubMed] [Google Scholar]
- Presscot GW. 1962. Algae of western great lakes area. USA: W.M.C. Brown Company. [Google Scholar]
- Ramirez RJJ, Diaz CA. 1994. Caracterización limnologica yestrutura de la comunidad fitoplanctonica en la Laguna Del Parque Norte, Medellin, Colombia. Hoehnea 21: 7–28. [Google Scholar]
- Randhawa MS. 1959. Zygnemaceae. In Zygnemaceae. New York: Indian Council of Agricultural Research, New Delhi. [Google Scholar]
- Reynolds CS. 1992. Dynamics, selection and composition of phytoplankton in relation to vertical structure in lakes. Arch for Hydrobiol 35: 13–31. [Google Scholar]
- Robson BJ, Hamilton DP. 2003. Summer flow event induces a cyanobacterial bloom in a seasonal Western Australian estuary. Mar Freshw Res 54: 139–151. [CrossRef] [Google Scholar]
- Rothhaupt KO. 2000. Plankton population dynamics: food web interactions and abiotic constraints. Freshw Biol 45: 105–109. [CrossRef] [Google Scholar]
- Shannon CE, Wiener W. 1949. The Mathematical Theory of Communication. Urbana: University of Illinois. [Google Scholar]
- Shenoy DM, Kurian S, Shirodkar G, Uskaikar H, Gauns M, Naqvi SW. 2021. Impact of physical processes on oxygen loss and production of hydrogen sulphide and methane in a tropical freshwater reservoir. Environ Sci Pollut Res 1–13. [Google Scholar]
- Shi X, Li S, Zhang M, Liu C, Wu Q. 2020. Temperature mainly determines the temporal succession of the photosynthetic picoeukaryote community in Lake Chaohu, a highly eutrophic shallow lake. Sci Total Environ 702: 134803. [PubMed] [Google Scholar]
- Silva CA Da Train S, Rodrigues LC. 2005. Phytoplankton assemblages in a Brazilian subtropical cascading reservoir system. Hydrobiologia 537: 99–109. [CrossRef] [Google Scholar]
- Smith GM. 1924. Ecology of the plankton algae in the Palisades Interstate Park, including the relation of control methods to fish culture. Roosevelt Wildlife Bull 2: 95–195. [Google Scholar]
- Soares MCS, Maria MI, Marinho MM, Azevedo SMFO, Branco CWC, Huszar VLM. 2009. Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: physical factors, nutrients and grazing effects. Aquat Microb Ecol 57: 137–149. [CrossRef] [Google Scholar]
- Sommer U, Gliwicz Z, Lampert W, Duncan A. 1986. The PEG-model of seasonal succession of planktonic event in fresh waters. Arch Hydrobiol 106: 433–471. [Google Scholar]
- Tian C, Lu X, Pei H, Hu W, Xie J. 2013. Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environ Monit Assess 185: 2627–2645. [PubMed] [Google Scholar]
- Tucci A, Sant Anna CL. 2003. Cylindrospermopsis raciborskii (Woloszynska) Seenayya & Subba Raju (Cyanobacteria): variaçao semanal e relaçoes com fatores ambientais em um reservatorio eutrofico, Sao Paulo, SP, Brasil. Rev Bras Botanica 26. [Google Scholar]
- Tyler P, Vyverman W. 1996. The microbial parket-place − trade-offs at the chemocline of meromictic lakes. Progr Phycol Res 11: 325–370. [Google Scholar]
- Vanni MJ, Temte J. 1990. Seasonal patterns of grazing and nutrient limitation of phytoplankton in a eutrophic lake. Limnol Oceanogr 35: 697–709. [CrossRef] [Google Scholar]
- Wang S, Qian X, Han BP, Luo LC, Hamilton DP. 2012. Effects of local climate and hydrological conditions on the thermal regime of a reservoir at Tropic of Cancer, in southern China. Water Res 46: 2591–2604. [PubMed] [Google Scholar]
- Wanganeo A. 2010. Manasbal Lake Kashmir-phytoplankton photosynthesis, nutrient dynamics and trophic status. Utpal Pub. [Google Scholar]
- Ward B, Rees AP, Somerfield PJ, Joint I. 2011. Linking phytoplankton community composition to seasonal changes in f-ratio. ISME J 5: 1759–1770. [PubMed] [Google Scholar]
- Watson SB, McCauley E, Downing JA. 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr 42: 487–495. [Google Scholar]
- Wichelen J V, Vanormelingen P, Codd GA, Vyverman W. 2016. The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 55: 97–111. [PubMed] [Google Scholar]
- Xiao LJ, Wang T, Hu R, et al. 2011. Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large canyon-shaped reservoir. Water Res 45: 5099–5109. [CrossRef] [PubMed] [Google Scholar]
- Yang JR, Lv H, Isabwe A, et al. 2017. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res 120: 52–63. [PubMed] [Google Scholar]
- Yildiz S, Altindag A, Ergonul MB. 2007. Seasonal fluctuations in the zooplankton composition of a eutrophic lake: Lake Marmara (Manisa, Turkey). Turk J Zool 31: 121–126. [Google Scholar]
- Zhang H, Jia J, Chen S, et al. 2018. Dynamics of bacterial and fungal communities during the outbreak and decline of an algal bloom in a drinking water reservoir. Int J Environ Res Public Health 15: 361. [CrossRef] [Google Scholar]
- Zhang H, Zhou X, Wang L, Wang W, Xu J. 2018. Concentrations and potential health risks of strontium in drinking water from Xi'an, Northwest China. Ecotoxicol Environ Saf 164: 181–188. [PubMed] [Google Scholar]
- Zhu W, Wan L, Zhao L. 2010. Effect of nutrient level on phytoplankton community structure in different water bodies. J Environ Sci 22: 32–39. [CrossRef] [Google Scholar]
Cite this article as: Ahmed A, Gauns M, Shenoy DM, Kurian S, Naik H, Naqvi SWA. 2021. Phytoplankton dynamics in a seasonal stratified reservoir (Tillari), Western India. Ann. Limnol. - Int. J. Lim. 57: 20
All Tables
Variation of predominant species of phytoplankton in Tillari (Predominant indices).
All Figures
 |
Fig. 1 Map showing sampling location (Tillari reservoir). |
| In the text | |
 |
Fig. 2 Vertical profile of dissolved oxygen, temperature and chlorophyll a ( a: February2011 b: May, 2010 and c: August 2011) and nitrate, phosphate and silicate. |
| In the text | |
 |
Fig. 3 Temporal variation in chlorophyll a:Dark filled bars represent integrated Chl a and dark line represents surface Chl a. |
| In the text | |
 |
Fig. 4 Vertical profile of phytoplankton cell abundance in Tillari reservoir. |
| In the text | |
 |
Fig. 5 Vertical profile of different phytoplankton taxonomic groups. |
| In the text | |
 |
Fig. 6 Vertical distribution of phytoplankton cells in different strata, A: during stratification period B: during mixing period, Chlor: chlorophytes, Char: charophytes, Cyan: cyanobacteria, Diat: diatom. |
| In the text | |
 |
Fig. 7 Temporal variation in phytoplankton diversity index. |
| In the text | |
 |
Fig. 8 Relationship between surface nitrate and chlorophyll concentration. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


