| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 54, 2018
|
|
|---|---|---|
| Article Number | 23 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/limn/2018012 | |
| Published online | 20 June 2018 | |
Research Article
Isotope stratification of meromictic Lake Trekhtzvetnoe at the White Sea coast (Russia)
1
Department of Geography, Lomonosov Moscow State University,
Moscow, Russia
2
School of Basic Sciences, North-West University Vaal Campus,
Noordbrug, South Africa
3
Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry, Russian Academy of Science,
Moscow, Russia
4
N.A.Pertsov White Sea Biological Station, Lomonosov Moscow State University,
Moscow, Russia
5
Centre for Geo-Environmental Research and Modelling (GEOMODEL) at St. Petersburg University,
St. Petersburg, Russia
* Corresponding author: vasilch_geo@mail.ru
Received:
12
August
2017
Accepted:
7
March
2018
Postglacial isostatic uplift of the coastal zone resulted in the formation of isolated lakes along the shores of White Sea developed into permanently stratified (meromictic) lakes. On the basis of monitoring the vertical distribution of selected water quality parameters including temperature, salinity, concentration of major ions, microbial activity, dissolved oxygen and hydrogen sulfide, seasonal changes in the structure of the stratified water column are explored and governing factors identified. Lake Trekhtzvetnoe has a strong vertical stratification with seasonal mixing being confined to the upper part of the water column (mixolimnion). Сhemical water composition in the mixolimnion reflects the influence of precipitation with the underlying chemocline being defined by sharp gradients of salinity, dissolved oxygen and hydrogen sulphide levels. We found strong stable isotope stratification of water column in winter-early spring seasons of 2013, 2015 and 2016 and in the early autumn of 2015. The lowest isotope values were obtained near the surface in the mixolimnion. There was a gradual increase of isotope values towards the chemocline reaching the maximum in the bottom layer (monimolimnion). It was found that water in the mixolimnion was isotopically depleted in winter as a result of ice formation and isotopically enriched in the early autumn due to evaporation. Obvious desalination of mixolimnion and upper chemocline from 2012 to 2016 was possibly caused by the increase of precipitation and freshwater inflow in lake supply. Monimolimnion is a stable layer of high salinity, and it has enriched isotope composition that corresponds with meromictic structure of water column.
Key words: White Sea / separating lakes / meromixis / stratification / stable isotopes
© EDP Sciences, 2018
1 Introduction
Most of the White Sea coast still rises isostatically after the last glacial period gradually leading to the separation of numerous small gulfs and coastal channels from the sea and the subsequent formation of small lakes. In the vicinity of the White Sea Biological Station of MSU (WSBS), the rate of the uplift is about 40 cm per century (Romanenko and Shilova, 2012). This, together with meandering shoreline and abundance of islands and cross-country terrain provides favourable conditions for separating the bays from the sea and forms many water bodies. Some lakes present different stages of isolation from the sea beginning with the lagoon with tidal fluctuations to the meromictic reservoirs with a fresh upper layer and strong stratification (Krasnova et al., 2014). The influx of salt water stimulates the process of bacterial sulfate reduction resulting in hydrogen sulfide in the lower water layer and proliferation of anaerobic phototrophic microorganisms, including green sulfur bacteria in the redox zone. Colored microbial water layers in the redox zone are indicators of the stage of lake evolution. Red beds with cryptophyte algae bloom indicate early stages of the reservoir development, green layers with green sulfur bacteria indicating advanced stages. The development of microbial color layers influences the vertical hydrological structure by depriving light of most of the water column (Pantyulin and Krasnova, 2011; Krasnova et al., 2013, 2014). These newly formed lakes provide the opportunity to study the formation of anaerobic conditions in the hydrosphere and the subsequent interactions between marine and fresh-water based fauna as well as a variety of associated biogeochemical processes in water and sediments. The lake separated from the sea is forcing the stratification of water column and transformation to typically meromictic structure. This is accompanied by development of stagnation in the bottom layer which sharply impairs ecological conditions of the lake.
The typical hydrological structure of stratified lakes consists of an upper layer mixolimnion 1–1.5 m thick and a monimolimnion divided into two layers: the upper photic aerobic and lower aphotic anaerobic layers. Between the main layers there is narrow transition zone with sharp gradients of physical and chemical parameters: a pycnocline on the border of freshened and salt layers, and a redox zone between aerobic and anaerobic layers. Oxygen and hydrogen isotope composition of water column of stratified lakes often shows three-layered structure as following: the mixolimnion layer which has an isotopic signal coherent with the values for local precipitation, the chemocline having the δ18О and δ2H values increasing towards the monimolimnion where they reach their maximum.
As 18O is a minor constituent of the water molecule, it imparts conservative properties of meteoric waters through the hydrological cycle. In conjunction with salinity, the isotopic ratios may be used to detect any dilution resulting from mixing of isotopically distinctive components, for example sea-water with continental waters. The isotopic composition may also provide some insight into any physicochemical process which can modify the isotopic ratio. For example, water in isolated lakes is exposed to evaporation, which leads to isotopic enrichment and to an offset of the δ18O and δ2H values below the meteoric water line (GMWL) defined by the equation: δ2H = 8δ18O + 10‰ (Craig, 1961).
Case studies show that isotope stratification of water may be caused by different factors. For the Black Sea, enrichment in deuterium and 18O isotopes for the deep waters (depth over 500 m) compared with the surface layer is explained by mixing of surface water with the Lower Bosporus Current inflow (Dubinin et al., 2014). For seasonally isolated marine basin in the Canadian High Arctic, the stable oxygen and hydrogen isotope data coupled with hydrochemical data showed the brine rejection during ice formation as a mechanism for the generation of hypersalinity (Dugan and Lamoureux, 2011).
In meromictic lakes Sophia and Garrow studied on the Little Cornwall Island, Canadian Arctic, mixolimnion zone is characterized by low chloride concentration and δ18О values close to that of precipitation. In the monimolimnion zone (20–47 m depth) δ18О values reach a maximum at a salinity which is 2–2.5 times higher than in the adjacent ocean (45–50 g/L). The hyper-saline water enriched with isotopes could have been formed in the monimolimnion of both lakes as a result of crenogenic salinization that started when both lakes became isolated from the sea following gradual uplift of the land over 4000 years ago. Stable isotope and salinity stratification in Lake Sophia caused by infiltrating brines from the ocean subterranously through the unfrozen zone into the lake also (Page et al., 1984; Ouellet et al., 1989).
Studies of shallow stratified permafrost-thaw lakes (depth 2.7–4 m) in the Canadian Subarctic showed that density stratification of water is especially developed during winter when the lakes are covered with ice. The concentration of dissolved oxygen in these lakes then decreases to zero across the entire stratified water column because of reducing conditions, and lack atmospheric oxygen. At high concentrations of organic matter this results in methane production during winter (Deshpande et al., 2015).
This study investigates stable isotope stratification of meromictic Lake Trekhtzvetnoe. In the study area it is one of the deepest coastal lakes separated from the White Sea. Long-term detailed studies of hydrological parameters of Lake Trekhtzvetnoe revealed that it is characterized by stable stratification of water column, and anomalously high hydrogen sulfide concentration that makes it unique among known meromictic reservoirs. The objective is to elucidate the mechanisms that result in the formation of observed isotope composition of mixolimnion, chemocline and monimolimnion. These data provide a new addition to the modern isotope dataset of meromictic lakes.
2 Materials and methods
2.1 Study site and sampling
Trekhtzvetnoe Lake is located in the Rugozerskaya Bay of the White Sea (66°35,53′N, 32°59,97′E), near WSBS of Moscow State University (Fig. 1). The size of the lake is 340 m × 150 m, the maximum depth is 8 m. It is separated from the White Sea by an isthmus that only allows for minimal drainage of lake water into the sea during summer (max. 1 l/s) with no flow occurring during winter. Consequently, no tidal fluctuations of the water level in the lake are observed.
The bottom of the lake has a funnel form (Fig. 2) that together with a lack of lake-sea water exchange resulted in stable stratification of water column and formation of a typical meromictic structure with upper freshwater layer and salty monimolimnion separated by chemocline (Fig. 2).
For the isotopic analysis 50 ml water samples were collected at every 0.5 m from surface to bottom in March 2012, 2013 and 2016, in January and September 2015, using the Whale Premium Submersible Pump GP1352 (USA). Samples were sealed and stored at a temperature of about 2 °C.
 |
Fig. 1 Location of Trekhtzvetnoe Lake in the vicinity of WSBS. |
 |
Fig. 2 Average position of mixolimnion, chemocline and a monimolimnion borders in the Trekhtzvetnoe Lake during 5 years of studies (2012–2016) based on salinity distribution along the cross (AB) and longitudinal (CD) profiles. |
2.2 Meromictic structure of Trekhtzvetnoe Lake observed in 2011–2016
The Russian name “Trekhtzvetnoe” means “three-color” because of the impressive differences in color of its three layers determined by sampling of water: the upper, fresh mixolimnion from the surface to 1–1.5 m depth is brownish due to humic substances entering the lake from the surrounding swamp. From 1–1.5 to 2.5–3 m there is chemocline with a steep salinity gradient. Underneath the water is salty. The middle water layer from 1.5 to 1.75–1.9 m is aerobic; the border between aerobic and anaerobic layers can be observed at a depth of 1.75–1.9 m depending on the seasonal water level fluctuations. At the same time, this boundary is a chemocline or redox zone, yet all through the year, its color remains bright green as a result of the growth of green sulfur bacteria (Kharcheva et al., 2013; Krasnova et al., 2015). Below the redox zone, there is a hydrosulfide water layer colored in lemon yellow, turbid due to sulfur crystals. When lifting to the air gas bubbles appear in the water samples (presumably methane). Among the known lakes separated from the White Sea the Lake Trekhtzvetnoe best meets the meromictic concept (Vasil’chuk et al., 2016). The vertical stratification was constant throughout the year and over the six years (2011–2016) of observation.
2.2.1 Temperature and salinity
Vertical temperature profiles of the lake suggest the existence of three layers across all seasons. These include the following: (1) a surface layer affected by wind-related mixing, (2) a subjacent layer of temperature transition layer (thermocline) and (3) a lower layer where density-driven convection is absent. Generally, in winter the water temperature below the ice is about 0 °C. In winter 2012, the temperature of lake water under the ice cover was −0.4 °C due to higher salinity caused by preceding intrusion of sea water in autumn 2011.
After ice melting, the temperature of the upper water layer gradually increases to summer maxima of about 14–16 °C, some 2 m above the thermocline. In early September, the bottom boundary of the thermocline drops to 3–4 m depth below surface. The surface water layer gradually cools down to 10–16 °C depending on weather conditions in late August to September. During this time a specific temperature depth profile is formed in the thermocline: it is warmer than the upper and the lower layers with temperature reaching up to 18 °C. In early spring, an inverse temperature profile develops. The temperature of the bottom water layer remains constant at 5.5–6.0 °C throughout the year (Fig. 3A).
The salinity of mixolimnion during a year does not exceed 0.8–1.8 psu, except for 2012 when it reached 5 psu (Fig. 3B). The salinity in the chemocline does not exceed 16 psu and in the monimolimnion it gradually declines from 14 to16‰ at the upper boundary to 20–23 psu at the bottom. In November 2011, a high tide coinciding with a wind surge pushed seawater into all coastal lakes, including the Trekhtzvetnoe Lake. This event was unique for a lake that was separated from the sea for many years. As a result, in winter 2012, the thickness of mixolimnion decreased to 1 m and the water turned brackish. In the next winter seasons, salinity of the surface layer decreased to 0.8–0.6 psu and in winter of 2015 it did not exceed 0.4 psu. The water salinity in the monimolimnion (from 4 m depth to the bottom at 8 m), was about 20–23 psu, during both summer and winter.
Chemical composition of the lake water is characterized by chlorine and sodium dominance (Tabs. S1, S2). The sum of ions increases from 13 g/L (at a depth of 2 m) to 23 g/L at the bottom, but concentration of all major ions remains nearly constant throughout the year. The distribution of main ions and dissolved elements correlates with chloride concentration and is typical for water with intermediate salinity (Savenko et al., 2015). Vertical distribution of sulfates shows maxima at the depth of 2–4 m, and below this level, it decreases with depth, which is different from the vertical profile of all other ions and caused by bacterial oxidation of sulfides to elemental sulfur.
The penetration of dissolved oxygen into the lake is limited by the chemocline level at 1.75–1.9 m. During winter seasons ice cover prevents input of atmospheric oxygen into the lake and while the decomposition of organic matter consumes the available remainder of DO, anoxic conditions gradually develop which allow for the subsequent accumulation of hydrogen sulfide up to 630 mg/l in monimolimnion (Kokryatskaya et al., 2013; Losyuk et al., 2015) that is about 2–3 times higher than in the Norwegian Framvaren Fjord where one of the highest level of H2S was measured (Millero, 1991; Behnke et al., 2006).
Light penetration in this lake is limited by the upper 1.5 m. The middle green layer is muddy because of the huge number of bacteria, and it completely absorbs light; so most of the water column in this lake stays in the aphotic zone. Our studies showed oxygen isotope stratification of Lake Trekhtzvetnoe water column in March 2012 (Lisitzin et al., 2013). Surface layer from 0 to 1 m was characterized by the lowest δ18О values from −10.8 to −10.2‰; at 1.5 a shift to δ18О value of −7.1‰ was observed with gradual increase to +2.1‰ to the bottom. In the vicinity of WSBS some lakes on the initial stage of separation from the sea were characterized by rather uniform vertical distribution of δ18О and salinity close to that of the sea. Uniform δ18О profiles were also typical for the shallow lakes completely separated from the sea and turned to fresh (Lisitzin et al., 2013). It is found that applying the stable isotope data together with temperature and salinity distribution in water column may appear as an effective method in defining the water sources feeding lakes isolated from the sea.
 |
Fig. 3 Annual variability of temperature (A) and salinity (B) in the Lake Trekhtzvetnoe in 2011–2016. |
2.3 Analytical measurements
Isotope measurements were made in the Stable Isotope Laboratory of the Faculty of Geography at the Lomonosov Moscow State University by isotope equilibrium method on CF-IRMS (Finnigan Delta-V). Equilibration for CO2 took place during 24 h in 24 °C temperature tray, for 2H during 40 min with Pt-catalizator. For calibration international standards (V-SMOW, GISP, SLAP) were used.
All δ2H and δ18O values were expressed relative to Vienna Standard Mean Ocean Water (VSMOW) in parts per thousand (%),
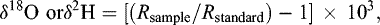 where R is the ratio of 2H to 1H atoms or 18O to 16O atoms in the sample and the standard VSMOW.
where R is the ratio of 2H to 1H atoms or 18O to 16O atoms in the sample and the standard VSMOW.
Anomalously high values of δ2H in the monimolimnion of Lake Trekhtzvetnoe were obtained for several sampling dates (March 2013 and January 2015). Repeated measurements carried out 3 weeks later showed no anomalously high 2H levels and a much reduced smell of hydrogen sulfide. Perhaps, high δ2H values obtained in the monimolimnion are explained by instrumental errors (mass spectrometer Delta V) that occur if isotope ratios are measured as in water as in the dissolved H2S found at high concentrations in the monimolimnion that could disrupt the chromatogram.
To compare values obtained on different isotope analyzers some samples (March 2016 and September 2015) were also measured at the Saint Petersburg State University Resource Center for Geo-Environmental Research and Modeling (GEOMODEL) on the Isotopic Water Analyzer Picarro L-2120i (Picarro INC., USA). Picarro analyzers use the method of Wavelength Scanned Ring Down Spectroscopy (WS-CRDS) providing precise, quantitative measurements even in samples with trace gas concentration. Measurement precision was ±0.1‰ for δ18O and ±1‰ for δ2H.
3 Results and discussion
3.1 Oxygen and hydrogen isotopes
The contrasting distribution of oxygen and hydrogen isotope values observed in 2012–2016 corresponds with meromictic structure of Trekhtzvetnoe Lake (Tabs. S1 and S2, Fig. 4), except for March, 2012 when water column stratification was slowly indistinct that most likely caused by the input of a large volume of seawater in November 2011. The δ18О–δ2H ratio slope was 5.54 with R2 = 0.97 (Fig. 5, Tab. S3) for whole water column that was close to the sea water. In March 2013, in the mixolimnion δ18О values varied from −9 to −11.8‰ and δ2H values − from −68.9 to −91.5‰ (Tab. S1). In the chemocline (1.5–3 m depth) δ18О values increased to −5.8‰, δ2H values to −49.3‰. In the monimolimnion layer the δ18О values varied from −5.4 to −5.7‰.
In January 2015, the isotope stratification of water slightly changed. According to δ18О values distribution, the layer of mixolimnion had a thickness of about 1 m (δ18О varied from −12.1 to −11.1‰), in the chemocline to the depth of 3 m δ18О values raised to −5.5‰, in monimolimnion they varied in the narrow range between −4.7 and −5.2‰ (see Tab. S1). In 2013 and 2015, anomalously high values of δ2H were determined in monimolimnion (see Tab. S1, Fig. 4). In 2013, the δ2H values were 780–1100‰ (4–6.5 m depth) and in 2015 δ2H values ranged from 1600 to 2400‰ (3.5–7.6 m depth). In March 2016, the isotope composition of water in the mixolimnion (0–1 m) was quite depleted: δ18О values varied from −12.6 to −13.6‰, δ2H values − from −102 to −94‰. In the chemocline (1–3 m), the isotope values increase to −6.9‰ for δ18О and to −53‰ for δ2H. In the monimolimnion δ18О values varied in the range from −5.6 to −6.4 ‰, δ2H values − from −40 to −47‰. In September 2015, the meromictic isotope structure of the lake was less distinct − the range of oxygen isotope values between mixolimnion and monimolimnion was 4.8‰ while in winter months the range was 6.7–12.9‰ (see Tab. S2). Nevertheless, the mixolimnion to the depth of 1.8 m had δ18О values from −10.7 to −10.9‰, whereas the δ2H values ranged from −82 to −80 ‰. In the chemocline, the increase of isotope values was noted: to −7.1‰ for δ18О, to −54‰ for δ2H to the depth of 1.9–3.0 m. In the monimolimnion δ18О values varied from −6.1 to −7.3‰, δ2H values from −45 to −51‰.
It is revealed that in 2013, 2015 and 2016, the water of mixolimnion and chemocline had values of δ18О–δ2H ratio from 6.7 to 7.6 that is close to those for meteoric water. The water of monimolimnion in 2012, 2016 and 2015 (September) was characterized by values of δ18О–δ2H ratio from 2.5 to 5.6 with R2 from 0.31 to 0.58 that would indicate the lack of mixing between monimolimnion and upper layers of chemocline and mixolimnion (see Fig. 5, Tab. S3).
The d-excess values of the lake water in 2012–2016 varied from −59.2 to 10.4‰, the most values were in the range from −5 to 7‰ (see Tabs. S1 and S2, Fig. S1). These data are comparable with those obtained for lakes in Western Siberia (between 62 and 67°30′N) (Ala-aho et al., 2018) in winter-spring and indicates that even in high latitudes the lake water has a noticeable signal of evaporation.
The δ18О–δ2H diagrams show the large range of isotope values in the mixolimnion in March 2013 and 2016 (see Fig. 5), that is possibly caused by ice formation. The difference in isotope values between upper and lower layers in mixolimnion was about 2–3‰ for δ18О and about 20‰ for δ2H that indicates isotope fractionation factor for water-ice separation. Obviously, surface water is a source of ice formation and it is well known that the ice is always isotopically enriched compared to the original water. The difference between δ18O values of ice and water is usually between 2 and 2.6‰ (O’Neil, 1968; Lehmann and Siegenthaler, 1991; Macdonald et al., 1995). A difference of 4‰ was observed between ice and initial water during the experimental ice formation in closed system (Vasil’chuk, 2011). In 2013, the ice was characterized by the values of δ18O = −10.2‰, and δ2H = −74.4‰ that allows us to suppose, that surface water layer was the source for lake ice formation. Therefore, due to the ice formation the isotope stratification of water column in winter is more pronounced.
 |
Fig. 4 The δ18О and δ2H profiles in the water of Lake Trekhtzvetnoe, 2012–2016. |
 |
Fig. 5 The δ18O–δ2H plots for water column of the Trekhtzvetnoe Lake. |
3.2 The dynamics of δ18О–S ratio in 2012–2016
The additional approach for isotope stratification analysis of the lake water is δ18О – salinity ratio which is usually applied for the studies of sea water masses and their mixture. As salty water in Trekhtzvetnoe Lake originated from the White Sea and fresh water of mixolimnion – from precipitation and runoff, it is possible to use a theoretical mixing line of White Sea water and local fresh water (Fig. 6, line AB). The point A represents the White Sea water (mean salinity value is 30 eps, mean δ18O value is −2‰, according to (Pavlov et al., 2016)). In the study area the salinity of White Sea water is about 27–29 psu in winter and 24–25 psu in summer. The point B represents the average value of mean annual precipitation on Kandalaksha weather station (GNIP data) together with runoff of large rivers flowing into the White Sea (mean salinity value is 0 eps, mean δ18O value is −14‰).
However, for Trekhtzvetnoe Lake the best linear approximation of the figurative points is the line connecting water of the White Sea and a fresh water with δ18О value of −12‰ (“best fit mixing line” on the Fig. 6). The difference of 2‰ may be explained by evaporation from the lake surface. In 2012–2016, the obvious variations of δ18О–S ratio are noted in mixolimnion and chemocline and less noticeable − in monimolimnion. The deviation from the mixing line is expressed by a value of Δδ18О (Tab. S3): the negative Δδ18О values indicate the lower oxygen isotope values of water than at proportional dual-component mixture (mixing line AB). The positive Δδ18О values indicate the higher oxygen isotope values of water relative to the dual-component mixture.
 |
Fig. 6 δ18О–S ratio in Trekhtzvetnoe Lake in 2012–2016. AB − mixing line of White Sea water and continental fresh water (local precipitation and runoff). |
3.2.1 Mixolimnion (0–1–1.5 m)
In winter of 2012, the lake water was brackish and salinity of upper 0.5–1 m layer was 5.2 psu. At the same time the δ18O values were 1‰ lower relative to dual-component mixture. The negative Δδ18О values indicate cryogenic isotopic transformation of water due to ice formation. Approximate balance calculation for the upper 1.5 m water layer (considering brine squeezing and isotope depletion of water as well as equilibrium fractionating at slow ice formation) showed that initial salinity and δ18О of surface lake water would be equal to 3.7 psu and −10.5‰, respectively. The calculated δ18О value for ice would be −8.5‰ (Fig. S2a).
In winter of 2013, the δ18О value of lower layer of lake ice was −10.2‰ and the water under ice had a δ18О value of −11.8‰ (see Fig. S2b). These data may indicate fast ice formation as the isotope shift between ice and initial water (ε) was 1.6‰ that is lower than obtained for slow ice formation (O’Neil, 1968; Vasil’chuk, 2011). From 0 to 1 m depth, the δ18О values increased by 2.8‰ while the salinity increased only by 0.09 psu that also shows isotope depletion due to ice formation. The similar δ18О–S ratio in the mixolimnion was observed in winter 2015: δ18О values increased by 1‰, the salinity increased by 0.2 psu (Fig. S2c, Tab. S3). In 2016 the upper 1 m layer of water was fresh, the values δ18О increased downward by 1‰ (Fig. S2e), whereas the negative Δδ18О values indicate isotope depletion due to ice formation (see Tab. S3).
Thus, it is possible to assume that the upper layer of mixolimnion was isotopically depleted as a result of ice formation, and its desalination from 2012 to 2016 occurred due to precipitation and freshwater inflow from the catchment. In September 2015, the mixolimnion was characterized by δ18О values = −10.9 and −10.6‰ at salinity 0.4 eps, so the water was almost fresh and isotopically enriched compared to winter seasons, that possibly reflects the prevailing influence of summer precipitation.
3.2.2 Chemocline (1–1.5–2.5–3 m)
In the chemocline (2012), the δ18O values (from −7.5 to −6.1‰) and salinity (from 5.2 to 17.1 psu) were close to the δ18О–S line (see Fig. S2a). In 2013, the δ18O values increased from −9 to −6.1‰, and salinity increased from 1 to 15.65 psu. Within 1.5–2 m, the δ18О–S points lie above the mixing line (see Fig. S2b); Δδ18О values at the depth 1–2 m exceeds 2 (see Tab. S3), which indicates stronger enrichment in isotopic composition relative to salinity. The same tendency was observed in winter 2015: δ18O values increased from −11.1 to −6.4‰, whereas the salinity increased from 1.9 to 14.3 psu. δ18O values were 1–2.5‰ higher than it is expected from dual-component mixture equation (see Fig. S2c, Tab. S3). This effect is often observed in sea water after melting of sea ice which is a source of rather fresh and isotopically enriched water (Bauch et al., 2016).
As it was mentioned above, the calculated value δ18О for lake ice in 2012 was −8.5‰ and in 2013 δ18О value of lower layer of ice was of −10.2‰.Therefore, the melting of lake ice could not cause the isotope enrichment of water in the chemocline. The possible reason of this effect would be isotope fractionation during photo- and a chemosynthesis which is active in the upper layer of chemocline characterized by high bacterial density on the border of aerobic and anaerobic zones.
In September 2015 and March 2016, the effect of isotope enrichment in the chemocline was not observed: in the layer between 1.5 and 2.5–3 m depth, the δ18О and salinity values were close to mixing line (see Fig. S2d and e). Comparison of Δδ18О variations in the chemocline in 2012–2016 shows considerable variability of values within the upper layer on the border of mixolimnion (depth 1–2 m) and lower variability within 2–2.5 m. In 2016, the upper part of chemocline was fresh as well as that of the mixolimnion due to precipitation and freshwater inflow from the catchment.
3.2.3 Monimolimnion (below 3 m)
The water in the monimolimnion is characterized by the highest values of salinity and δ18О. In 2012, 2013 and 2015, the salinity values varied from 17.5 to 22.9, the δ18О values varied from −6.9 to −3.8‰, and the figurative points of δ18О and S clustered close to the mixing line (see Fig. 5 and S2a–c). In September 2015 and March 2016, a shift from the mixing line was observed (see Fig. S2d and e): at the same salinity values (from 20.6 to 22.3 psu), the δ18О values became lower (from −6.2 to −5.6‰), the Δδ18О values in 2016 were the lowest (see Tab. S3). Comparison of Δδ18О variations in the monimolimnion in 2012–2016 shows the relative stability of values within the same depths. In 2012–2015 below 3 m Δδ18О values varied between 0.2 and −0.6, in 2016 Δδ18О values became much more negative (from −0.6 to −1.4‰) due to a decrease in δ18О values. In general, the monimolimnion is characterized by a high stability of physical and chemical parameters, including oxygen isotope composition. In contrast, the layer can be spatially non-uniform with slight variations of salinity and isotope values.
4 Conclusion
Overall, it can be concluded that the stable meromictic stratification in Lake Trekhtzvetnoe is caused by distinct differences in salinity and ion concentration, isotopic composition as well as dissolved oxygen and hydrogen sulfide concentrations. The water column of Lake Trekhtzvetnoe is characterized by stable isotope (18O and 2H) stratification. The lowest isotope values were obtained in the upper mixolimnion gradually increasing to the chemocline and reaching the maximum in the bottom layer (monimolimnion).
In winter seasons the oxygen isotope composition of water in the upper 0.5 m layer of mixolimnion was depleted due to ice formation. In winter seasons of 2013 and 2015, the water in the chemocline was isotopically enriched possibly due to isotope fractionation during photo- and chemosynthesis in the upper layer of chemocline where high bacterial density was reported. In September 2015 and March 2016, the δ18О–S ratio was close to mixing line of White Sea water - local fresh water that was possibly caused by influence of precipitation and freshwater inflow as well as a decrease in bacterial activity. The monimolimnion is a layer of stable high salinity, it exhibits enriched isotope composition; however, from 2012 for 2016, a slight decrease in isotope values was observed.
The obtained data contribute to the modern isotope dataset of polar meromictic lakes separated from the sea. Stable isotope composition of water column together with standard hydrological parameters provides valuable information for understanding the formation and evolution of these lakes. It is important for forecasting future changes in lake processes such as sea-level rise and coastal inundation.
Supplementary Material
Supplementary tables.
Access hereAcknowledgments
Authors gratefully acknowledge the support by the Director of the N.A. Pertsov White Sea Biological Station (WSBS) of Moscow State University, Dr. Mr. А.B. Tzetlin, the manager of WSBS, А.V. Savchenko, and also Dr. D.A. Voronov, Dr. N.A. Demidenko, Dr. N.M. Kokryatskaya, Dr. A.N. Pantyulin, V.V. Sivonen, V.T. Kolbyko, A.S. Filippov, A.V. Kharcheva, V.P. Shevchenko, and to all staff of WSBS for their continuous support.
The research was financially supported by the Russian Scientific Foundation: Grant No 14-17-00155 and by Russian Foundation for Basic Research (RFBR) Grant No 16-05-00548а for the hydrophysical, hydrochemical and microbiological aspects and Grant No 14-27-00083-P for the isotope related research. Support for the research was provided by the Commission for Water Sustainability of the International Geographical Union.
References
- Ala-aho P, Soulsby C, Pokrovsky OS, Kirpotin SN, Karlsson J, Serikova S, Vorobyev SN, Manasypov RM, Loiko S, Tetzlaff D. 2018. Using stable isotopes to assess surface water source dynamics and hydrological connectivity in a high-latitude wetland and permafrost influenced landscape. J Hydrol 556: 279–293. [CrossRef] [Google Scholar]
- Bauch D, Cherniavskaia E, Timokhov L. 2016. Shelf basin exchange along the Siberian continental margin: modification of Atlantic Water and Lower Halocline Water. Deep-Sea Res I 115: 188–198. [CrossRef] [Google Scholar]
- Behnke A, Bunge J, Barger K, Breiner H-W, Stoeck VA, Stoec T. 2006. Microeukaryote community patterns along an O2/H2S gradient in a supersulfidic anoxic fjord (Framvaren, Norway). Appl Environ Microbiol 72: 3626–3636. [CrossRef] [Google Scholar]
- Craig H. 1961. Isotopic variations in meteoric waters. Science 133: 1702–1703. [CrossRef] [PubMed] [Google Scholar]
- Deshpande BN, MacIntyre S, Matveev A, Vincent WF. 2015. Oxygen dynamics in permafrost thaw lakes: anaerobic bioreactors in the Canadian subarctic. Limnol Oceanogr 60: 1656–1670. DOI:10.1002/lno.10126. [CrossRef] [Google Scholar]
- Dubinin AV, Dubinina EO, Demidova TP, Kokryatskaya NM, Rimskaya-Korsakova MN, Kosova SA, Yakushev EV. 2014. Stable isotope evidence for the Bottom Convective Layer homogeneity in the Black Sea. Geochem Trans 15, http://www.geochemicaltransactions.com/content/15/1/3. [CrossRef] [PubMed] [Google Scholar]
- Dugan HA, Lamoureux SF. 2011. The chemical development of a hypersaline coastal basin in the High Arctic. Limnol Oceanogr 56: 495–507. [CrossRef] [Google Scholar]
- Kharcheva AV, Krasnova ED, Voronov DA, Gorshkova OM, Patsaeva SV. 2013. Spectral-optical and physical and chemical characteristics of meromictic lakes of the Kandalaksha Gulf of the White Sea: geology of seas and oceans. In: Proceedings of XX International Scientific Conference (School) of Sea Geology, Moscow, Vol. III, pp. 261–265 (in Russian). [Google Scholar]
- Kokryatskaya NM, Krasnova ED, Titova KV, Losyuk GN. 2013. Formation of hydrogen sulfide defilement of the lakes separating from the sea (Kandalakshsky Bay, White Sea). In: Marine Biology, Geology, Oceanography − Interdisciplinary Research in Marine Stations, Conference Proceedings dedicated to the 75th anniversary of N.A. Perzov White Sea Biological Station (27 February − 1 March 2013, Moscow), KMK, Moscow, pp. 123–126 (in Russian). [Google Scholar]
- Krasnova ED, Pantyulin AN, Belevich TA, Voronov DA, Demidenko NA, Zhitina LS, Ilyash LV, Kokryatskaya NM, Lunina ON, Mardashova MV, Prudkovsky AA, Savvichev AS, Filippov AS, Shevchenko VP. 2013. Multidisciplinary studies of the separating lakes at different stage of isolation from the White Sea performed in March 2012. Oceanology 53: 639–642. DOI:10.1134/S0001437013050068. [CrossRef] [Google Scholar]
- Krasnova ED, Voronov DA, Frolova NL, Pantyulin AN, Samsonov T. 2014. Salt lakes separated from the White Sea. In: Proceedings, Special Issue: 1st Student Workshop on Ecology and Optics of the White Sea, pp. 8–22. DOI: 10.12760/02-2015-1-02. [Google Scholar]
- Krasnova ED, Kharcheva AV, Milyutina IA, Voronov DA, Patsaeva SV. 2015. Study of microbial communities in redox zone of meromictic lakes isolated from the White Sea using spectral and molecular methods. J Mar Biol Assoc UK 95: 1579–1590. [CrossRef] [Google Scholar]
- Lehmann M, Siegenthaler U. 1991. Equilibrium oxygen and hydrogen isotope fractionation between ice and water. J Glaciol 37: 23–26. [CrossRef] [Google Scholar]
- Lisitzin AP, Vasil’chuk YK, Shevchenko VP, Budantseva NA, Krasnova ED, Pantyulin AN, Filippov AS, Chizhova JN. 2013. Oxygen Isotope Composition of Water and Snow-Ice Cover of Isolated Lakes at Various Stages of Separation from the White Sea. Doklady Earth Sci 449: 406–412. DOI: 10.1134/S1028334X1304003X. [CrossRef] [Google Scholar]
- Losyuk GN, Kokryatskaya NM, Krasnova ED. 2015. Formation of hydrogen sulfide in isolated basins at the Karelian of the White Sea coast. EARSeL eProceedings 14: 49–54. DOI: 10.12760/02-2015-1-07. [Google Scholar]
- Macdonald RW, Paten DW, Carmack EC. 1995. The freshwater budget and under-ice spreading of Mackenzie River water in the Canadian Beaufort Sea based on salinity and measurements in water and ice. J Geophys Res 100: 895–919. [CrossRef] [Google Scholar]
- Millero FJ. 1991. The oxidation of H2S in Framvaren Fjord. Limnol Oceanogr 36: 1007–1014. [CrossRef] [Google Scholar]
- O’Neil JR. 1968. Hydrogen and oxygen fractionation between ice and water. J Phys Chem 72: 3683–3684. [CrossRef] [Google Scholar]
- Ouellet M, Dickman M, Bisson M, Pagé P. 1989. Physico-chemical characteristics and origin of hypersaline meromictic Lake Garrow in the Canadian High Arctic. Hydrobiologia 172: 215–234. [CrossRef] [Google Scholar]
- Page P. Ouellet M. Hillaire Marcel C, Dickman M. 1984. Isotopic analyses (18O, 13C, 14C) of two meromictic lakes in the Canadian Arctic Archipelago. Limnol Oceanogr 29: 564–573. [CrossRef] [Google Scholar]
- Pantyulin AN, Krasnova ED. 2011. Isolated basins on the White Sea shore: the new object for interdisciplinary studies. In: Proceedings of XIX International Scientific Conference (School)of Sea Geology, Moscow, Vol. III. pp. 241–245 (in Russian). [Google Scholar]
- Pavlov AK, Stedmon CA, Semushin AV, Martma T, Ivanov BV, Kowalczuk P, Granskog MA. 2016. Linkages between the circulation and distribution of dissolved organic matter in the White Sea, Arctic Ocean. Cont Shelf Res 119: 1–13. [CrossRef] [Google Scholar]
- Romanenko FA, Shilova OS. 2012. The postglacial uplift of the Karelian coast of the White Sea according to radiocarbon and diatom analyses of lacustrine boggy deposits of Kindo Peninsula. Doklady Earth Sci 442: 242–246. DOI: 10.1134/S1028334X12020079. [CrossRef] [Google Scholar]
- Savenko AV, Demidenko NA, Savvichev AS, Pokrovsky OS. 2015. Distribution of major ions and dissolved trace elements in meromictic water reservoirs of Kandalaksha Bay of the White Sea. Geology of seas and oceans. In: Proceedings of XXI International Conference on Marine Geology, Moscow, Vol. III, pp. 271–275. (in Russian). [Google Scholar]
- Vasil’chuk YK. 2011. Experimental study of isotopic fractionation in water during congelation ice formation. Earth’s Cryosphere 3: 51–55. (in Russian). [Google Scholar]
- Vasil’chuk YK, Frolova NL, Krasnova ED, Budantseva NA, Vasil’chuk AC, Dobrydneva LV, Efimova LE, Terskaya EV, Chizhova JN. 2016. Water Isotopic-Geochemical Composition in the Trekhtsvetnoe Meromictic Lake on the White Sea Coast. Water Res 5: 828–838. DOI: 10.1134/S0097807816050110. [CrossRef] [Google Scholar]
Cite this article as: Vasil’chuk YK, Frolova NL, Kasimov NS, Winde F, Budantseva NA, Chizhova JN, Efimova LE, Krasnova ED, Terskaya EV, Tokarev IV, Vasil’chuk AC. 2018. Isotope stratification of meromictic Lake Trekhtzvetnoe at the White Sea coast (Russia). Ann. Limnol. - Int. J. Lim. 54: 23
All Figures
 |
Fig. 1 Location of Trekhtzvetnoe Lake in the vicinity of WSBS. |
| In the text | |
 |
Fig. 2 Average position of mixolimnion, chemocline and a monimolimnion borders in the Trekhtzvetnoe Lake during 5 years of studies (2012–2016) based on salinity distribution along the cross (AB) and longitudinal (CD) profiles. |
| In the text | |
 |
Fig. 3 Annual variability of temperature (A) and salinity (B) in the Lake Trekhtzvetnoe in 2011–2016. |
| In the text | |
 |
Fig. 4 The δ18О and δ2H profiles in the water of Lake Trekhtzvetnoe, 2012–2016. |
| In the text | |
 |
Fig. 5 The δ18O–δ2H plots for water column of the Trekhtzvetnoe Lake. |
| In the text | |
 |
Fig. 6 δ18О–S ratio in Trekhtzvetnoe Lake in 2012–2016. AB − mixing line of White Sea water and continental fresh water (local precipitation and runoff). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


