| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 25 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/limn/2021023 | |
| Published online | 25 November 2021 | |
Research Article
Impact of acute fonofos exposure on skeletal muscle of zebrafish: Histopathological and biometric analyses
1
Sakarya University, Faculty of Arts and Sciences, Department of Biology, 54050 Serdivan-Sakarya, Turkey
2
Ege University, Faculty of Science, Department of Biology, 35100 Bornova-Izmir, Turkey
* Corresponding author:: sezgiarman@gmail.com
Received:
30
July
2021
Accepted:
1
November
2021
It is widely known that pesticides generally do not show target specificity, and off-target species are strikingly affected by these chemicals. In the current work, histological changes in skeletal muscles of zebrafish (Danio rerio) caused by fonofos, an acetylcholinesterase (AChE) inhibitor organophosphate insecticide, were examined. Zebrafish were treated with 1 mg/L, 2 mg/L and 4 mg/L of fonofos for 96 hours. Skeletal muscle samples were removed from the pectoral region and embedded in paraffin. Sections were stained with Mayer's Hematoxylin and Eosin, Gomori's Trichrome and Periodic Acid Schiff techniques. Histopathological alterations were investigated by light microscopy. Fibrosis, intramyofibrillar vacuoles, disintegrated myofibrils, splitting of myofibers, atrophic and disappeared fibers, histoarchitectural loss, necrosis and progressive decrement in glycogen content were noted. Muscle fiber diameter measurements were also performed. Statistical analysis showed that measured fiber diameters of all fonofos exposed groups were significantly different from the control group, and they decreased in a concentration-dependent manner. These results suggested that fonofos caused significant myoarchitectural impairments in non-target freshwater zebrafish.
Key words: Organophosphate / insecticide / skeletal muscle / histopathology / Danio rerio
© EDP Sciences, 2021
1 Introductıon
Worldwide pesticide usage is still a big dilemma since it serves many advantages on agricultural crop productivity while it also causes considerable hazards to the health of farmers, off-target vertebrate species, and the environment. Although correctly used pesticides prevent up to 40% of crop loss (Richardson, 1998), it is also noted that they gave rise to annually at least 20.000 workers' death (Rahman, 2013). Pesticides are at the top of the most hazardous environmental pollutants list with metals and other organic compounds (Scott and Sloman, 2004). Numerous works reveal that vertebrates are strikingly affected by pesticide exposure (Kwon et al., 2004; Bonfanti et al., 2018; Chen et al., 2019; Al-Ghanim et al., 2020; Aliomrani et al., 2021). It is estimated that less than 0.1% of pesticides applied to crop fields reach the primary target, with the remainder polluting the soil, water resources and air inhabited by various non-target species (Pimentel and Levitan, 1986; Arias-Estévez et al., 2008).
Insecticides have one of the biggest market shares among pesticides. They are used to control approximately 9.000 species of insects and mites responsible for 14% of crop loss worldwide (Pimentel, 2009; Zhang et al., 2011). Their chemical structure is substantially organophosphate (OP), and OPs are considered the most toxic pesticides to vertebrates (Shadnia et al., 2005; Lukaszewicz-Hussain, 2010). Their mode of action is based on acetylcholinesterase (AChE) inhibition. When the AChE is inhibited, the neurotransmitter acetylcholine (ACh) can not be broken down in nerve synapses and muscular junctions. This case causes accumulation of ACh at these sites that lead to persistent stimulation of the muscle that is resulted in excessive stimulation and even death (Roex et al., 2003).
Several studies are available in the literature revealing OP insecticides adversely affect aquatic organisms via diverse pathways (reviewed in Sidhu et al., 2019). OP insecticides induced AChE inhibition (Üner et al., 2006), histopathology (Pugazhvendan et al., 2009), neurotoxicity (Sandoval-Herrera et al., 2019), behavioral changes (Singh et al., 2009), oxidative stress (Monteiro et al., 2006), developmental toxicity (Pamanji et al., 2015) and genotoxicity (Kumar et al., 2010) in various fish species.
Fonofos (O-ethyl S-phenyl ethylphosphonodithioate), with the trade name Dyfonate, is an OP insecticide applied to soil to prevent various worms that damage mainly corn, and also sugarcane, peanuts and tobacco (Mahajan et al., 2006; US EPA, 2008). Our knowledge regarding the adverse effects of fonofos is very limited.
Zebrafish (Danio rerio) is a unique vertebrate model for its various features. Firstly, its small size presents many advantages such as easy husbandry, reduced housing space, required low quantities of experimental solutions and low volume of toxic waste (Hill et al., 2002; 2005). Scientists have well studied the morphological, biochemical, and physiological processes of all life stages of zebrafish from zygote to adult (Hill et al., 2005). These benefits make zebrafish an excellent material for toxicological researches.
Skeletal muscles play essential roles such as contraction, moving, body posture, balance, protecting internal organs and glycogen storage. Recent studies have also proved that fish muscle is an immunologically active organ (Valenzuela et al., 2017). Besides, it is an important source of human nutrition. Skeletal muscle is known to be the target tissue of various xenobiotics (Gupta et al., 2014). The present study aimed to reveal whether fonofos causes qualitative and quantitative alterations on the skeletal muscle of zebrafish.
2 Materıals and methods
2.1 Animal husbandry
Adult zebrafish (3–4 months old) were maintained in glass aquaria with dechlorinated aged tap water at 26 ± 2 °C. Fish were kept under natural photoperiod for two weeks before the experiment, and they were fed with Artemia sp. twice a day.
2.2 Experimental design
Fonofos (99.5%) (CAS No: 944-22-9) and dimethyl sulfoxide (DMSO) (≥99.5%) were purchased from Sigma-Aldrich. The stock solution was prepared by dissolving fonofos in 0.1% DMSO. Treatment concentrations were diluted from the stock solution. A solvent control (0.1% DMSO) and three exposure groups (1 mg/L, 2 mg/L and 4 mg/L) were prepared. Five fish were used for each group. A static test system was conducted; fish were not fed, and the test solutions were not renewed for 96 h.
2.3 Histopathology and biometry
Fish were euthanized in 250 mg/L of tricaine methanesulfonate (MS-222) solution (Wang et al., 2020). Skeletal muscle samples were removed from the pectoral region. They were fixed in Bouin's fluid for 24 h at room temperature, dehydrated in ethanol, treated with xylol and embedded in paraffin. 5 μm-thick serial sections were stained with Mayer's Hematoxylin and Eosin (H&E) and Gomori Trichrome (GT) to observe general muscle histology, and they were also stained with Periodic Acid-Schiff (PAS) to detect glycogen content. Images were taken with Zeiss Axio Scope A1 equipped with Zeiss Axiocam ERc5s.
Biometric measurements were performed with oblique or transversally sectioned fibers, and diameters were measured according to the minimum diameter method of morphometry (MDM) to detect any changes in myofiber diameters described by Mars and Gregory (2014). 1800 fibers were analyzed for each experimental group. 20 diameters were measured in three random fields of 10 sections from three randomly selected individuals. Leica DM500 microscope with the software program Leica Application Suite (LAS) V4.9 were used for the measurements.
2.4 Statistics
Statistical analyses were performed with SPSS (Version 20). After the priority Shapiro-Wilk normality and Levene homogeneity tests, Kruskal-Wallis H was conducted to compare skeletal myofiber sizes between the control and the treatment groups. The significance level was set at p < 0.001.
3 Results
Normal skeletal muscle histology was observed in the solvent control samples. The striated appearance of myofibers and peripherally located nuclei were observed. Glycogen storages were noticed with magenta color in PAS-stained sections (Fig. 1).
No mortality was detected during the experiment. Some of the exposed individuals exhibited erratic swimming movements and spinal curvature. Light microscopic examinations revealed that fonofos exposure gave rise to distinct lesions in the muscle tissues of zebrafish specimens in the experimental groups. These lesions were majorly similar in all three experimental samples; however, the severity of the lesions was in a concentration-dependent manner. General histoarchitecture was significantly broken down following the exposure to ascending concentrations of fonofos. 1 mg/L of fonofos treated group showed fibrosis (Fig. 2a,c), intramyofibrillar vacuoles, atrophic fibers (Fig. 2b), disintegrated myofibrils (Fig. 2c) and mild decrease in glycogen storage (Fig. 2d).
2 mg/L of fonofos exposed group samples exhibited general deformation of tissue integrity. Fibrous tissue formation and intramyofibrillar vacuoles (Fig. 3a) were common. While some myofibers were atrophic, some disappeared fibers were also noticed (Fig. 3b). Disintegrated myofibrils and splitting of muscle fibers were observed with distinct gaps among the fibers (Fig. 3c). PAS-stained sections proved progressive decrement in glycogen content (Fig. 3d).
4 mg/L of fonofos treated group showed a severe histoarchitectural loss. Prominent fiber splitting, atrophic fibers and disintegration of myofibrils were noted (Fig. 4a). Intramyofibrillar vacuoles (Fig. 4b,c) and necrosis (Fig. 4c) was noticed. PAS staining technique showed a significant decrease in muscular glycogen in the highest fonofos concentration group (Fig. 4d).
Mean values and standard errors of measured fiber diameters were given in Table 1. Statistical analyses showed that the fiber diameters of all treatment groups were significantly different from the control (Tab. 1). The diameters (Fig. 5a) decreased in a concentration-dependent manner (Fig. 5b) indicating fonofos-induced gradual muscular atrophy.
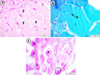 |
Fig. 1 Normal histological structure of the skeletal muscle of the control samples. (a) H&E. (b) GT. (c) PAS. F: muscle fiber; n: nucleus; *: glycogen storages; Ellipse: cross-striations. |
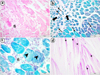 |
Fig. 2 Skeletal muscle sections of 1 mg/L of fonofos treated group. (a) Fibrosis (fi), H&E. (b) Intramyofibrillar vacuoles (thin arrows) and atrophic fibers (thick arrows), GT. (c) Fibrosis (fi) and disintegrated myofibrils (arrowheads), GT. (d) Decrease in glycogen storage (asterisks), PAS. |
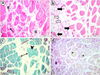 |
Fig. 3 Skeletal muscle sections of 2 mg/L of fonofos treated group. (a) Fibrosis (fi) and intramyofibrillar vacuole formation (arrow), H&E. (b) Deformation of general tissue integrity with atrophic myofibers (black arrows) and disappearance of some fibers (white arrows), H&E. (c) Splitting of muscle fibers (double-headed arrow) and disintegrated myofibrils (arrowhead), GT. (d) Progressive decrement in glycogen storage (asterisks), PAS. |
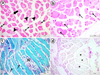 |
Fig. 4 Skeletal muscle sections of 4 mg/L of fonofos treated group. (a) Atrophic fiber (arrow), disintegrated myofibrils (arrowheads) and splitting of muscle fibers (double-headed arrow) accompanying with histoarchitectural loss, H&E. (b) Intramyofibrillar vacuoles (arrows), H&E. (c) Intramyofibrillar vacuoles (arrows) and necrosis (N), GT. (d) Severe decrement in glycogen content (asterisks), PAS. |
Measurements of skeletal muscle fiber diameters (μm) of zebrafish following exposure to fonofos for 96 h. Values indicate mean and standard error of measurements. An asterisk (*) indicates significant difference compared to the control (p < 0.001).
 |
Fig. 5 (a) Examples of fiber diameter (d) measurements of the control group (b) alterations in muscle fiber diameter measurements following 96 h fonofos exposed groups compared to the control. |
4 Dıscussıon
Pesticides have gained considerable notoriety as responsible for adverse effects on various non-target species (Lushchak et al., 2018). The current work showed that fonofos induced myotoxicity by causing striking histopathological alterations in the skeletal muscle of zebrafish. Muscular tissue was sensitive to fonofos treatment at the sublethal concentrations.
There are relatively few studies about muscle histopathology in fishes. Skeletal muscle toxicity caused by environmental contaminants available in the literature generally brought about similar lesions in fish. Two different freshwater fish species from pesticide polluted Lake Qarun were investigated and vacuolar degeneration in muscle bundles, atrophy, splitting of muscle fibers, and necrosis was observed in Tilapia zillii. In Solea vulgaris, degeneration in muscle bundles, vacuolar degeneration and edema between muscle bundles were noted (Mohamed, 2009). The skeletal muscles of Carassius gibelio, caught from Buyuk Menderes River polluted by several sources such as industrial and domestic wastes, fertilizers and pesticides, showed intermyofibrillar edema, vacuolization, dissociation of connective tissue, muscular atrophy and myofiber necrosi (Adalı and Koca, 2016).
Skeletal muscles are the targets of many chemicals due to their large portion in total body weight, high metabolic activity and various binding sites (receptors, neurotransmitters, enzymes, etc.); moreover, cholinergic and noncholinergic components of muscles are regulated by OPs (Gupta et al., 2014). Previous studies focused on skeletal muscle histopathology of fish treated with OP insecticides. Muscle tissues of diazinon exposed Tilapia nilotica exhibited ultrastructural changes such as swelling of the sarcoplasmic reticulum, loss of myofibrils extending over the entire length of the sarcomere, destruction in muscle structure, and abundance of phagocytic lysosomes (Sakr and Gabr, 1992). In the present work, fonofos-induced lesions as fibrosis, intramyofibrillar vacuoles, disintegrated myofibrils, atrophic myofibers, splitting of the fibers, histoarchitectural loss and necrosis were observed. Measurements also revealed progressive decrement in myofiber size. However, no significant histopathological alterations were reported in the muscles of Oreochromis niloticus exposed to fenitrothion (Benli and Özkul, 2010). Chlorpyriphos treatment brought about reduced myotome size, hypertrophy and vacuole formations in the myocytes of Xenopus laevis larvae (Colombo et al., 2005). In higher vertebrates, myopathic alterations were also stated following OP intoxication in the diaphragm, gastrocnemius and psoas muscles of rats (Karalliedde and Senanayake, 1989). Cisson and Wilson (1982) noted muscular atrophy in birds exposed to tricresyl phosphate and parathion. Fenthion induced edema, inflammation and necrosis in diaphragm tissues of rats (Büyükokuroğlu et al., 2008). It was revealed that OP intoxication induced atrophy and muscular necrosis in humans (Fukuhara et al., 1977). It was announced that skeletal muscle toxicity induced by AChE inhibitors might be related to excess free radical generation, lipid peroxidation, depletion of high-energy phosphates, high cytosolic Ca2+ degrees, mitochondrial damage, necrosis, apoptosis, and myopathy (Gupta et al., 2009).
On the other hand, PAS-stained sections suggested that acute fonofos treatment brought about muscular glycogen decrement in zebrafish. Glycogen decrement was reported as a common effect of pesticide exposure related to energy demands to eliminate the chemical and rapid catabolism of glycogen storages after toxic stress (Begum and Vijayaraghavan, 1999; Becker et al., 2009). Organochlorine pesticide endosulfan exposure gave rise to decreased muscle glycogen content through glycogenolysis in Anguilla anguilla (Gimeno et al., 1995). Moreover, a decrease in glycogen levels was noted in muscles of A. anguilla following lindane treatment (Ferrando and Andreu, 1991). It was revealed that glyphosate reduced muscle glycogen content in Leporius obtusidens (Glusczak et al., 2006). Clomazone also caused glycogen decrement in the muscle tissue of Rhamdia quelen (Crestani et al., 2006). Begum and Vijayaraghavan (1999) observed a gradual decrement in muscular glycogen content of Clarias batrachus exposed to Rogor insecticide. However, Sastry and Siddiqui (1984) noted increased glycogen content following quinalphos treatment in Channa punctatus. The chemical formulation of the pesticide and interspecies variations may alter the physiological responses to chemical stress.
The results indicated that besides their agricultural benefits, OP insecticides give serious harm to freshwater fish and threaten their lives. It is clear that ‘benefit’ must be thought of as a whole phenomenon consisting of both the human population's food requirements and all other species' health. So that eco-friendly techniques for agricultural developments are required to sustain the fate of the biosphere.
Acknowledgements
This study was presented as an oral presentation in International Marine and Freshwater Sciences Symposium (2018) held in Antalya-Turkey.
References
- Adalı Y, Koca YB. 2016. Effects of pollution on some tissues of fish collected from different regions of Buyuk Menderes River: a histopathological study. J Environ Protect Ecol 17: 477–487. [Google Scholar]
- Al-Ghanim KA, Mahboob S, Vijayaraghavan P, Al-Misned FA, Kim YO, Kim HJ. 2020. Sub-lethal effect of synthetic pyrethroid pesticide on metabolic enzymes and protein profile of non-target Zebra fish, Danio rerio. Saudi J Biol Sci 27: 441–447. [CrossRef] [PubMed] [Google Scholar]
- Aliomrani M, Mesripour A, Sayahpour Z. 2021. AChR is partly responsible in mice depressive-like behavior after Phosalone exposure. Neurotoxicol Teratol 84: 106957. [CrossRef] [PubMed] [Google Scholar]
- Arias-Estévez M, López-Periago E, Martínez-Carballo E, Simal-Gándara J, Mejuto JC, García-Río L. 2008. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric Ecosyst Environ 123: 247–260. [CrossRef] [Google Scholar]
- Becker AG, Moraes BS, Menezes CC, et al. 2009. Pesticide contamination of water alters the metabolism of juvenile silver catfish, Rhamdia quelen. Ecotoxicol Environ Saf 72: 1734–1739. [CrossRef] [PubMed] [Google Scholar]
- Begum G, Vijayaraghavan S. 1999. Effect of acute exposure of the organophosphate ınsecticide rogor on some biochemical aspects of Clarias batrachus (Linnaeus). Environ Res 80: 80–83. [CrossRef] [PubMed] [Google Scholar]
- Benli AÇK, Özkul A. 2010. Acute toxicity and histopathological effects of sublethal fenitrothion on Nile tilapia, Oreochromis niloticus. Pestic Biochem Physiol 97: 32–35. [CrossRef] [Google Scholar]
- Bonfanti P, Saibene M, Bacchetta R, Mantecca P, Colombo A. 2018. A glyphosate micro-emulsion formulation displays teratogenicity in Xenopus laevis. Aquat Toxicol 195: 103–113. [CrossRef] [PubMed] [Google Scholar]
- Büyükokuroğlu ME, Cemek M, Tosun M, Yürümez Y, Baş O, Yavuz Y. 2008. Dantrolene may prevent organophosphate-induced oxidative stress and muscle injury. Pestic Biochem Physiol 92: 156–163. [CrossRef] [Google Scholar]
- Chen L, Diao J, Zhang W, Zhang L, Wang Z, Li Y, et al. 2019. Effects of beta-cypermethrin and myclobutanil on some enzymes and changes of biomarkers between internal tissues and saliva in reptiles (Eremias argus). Chemosphere 216: 69–74. [CrossRef] [PubMed] [Google Scholar]
- Colombo A, Orsi F, Bonfanti P. 2005. Exposure to the organophosphorus pesticide chlorpyrifos inhibits acetylcholinesterase activity and affects muscular integrity in Xenopus laevis larvae. Chemosphere 61: 1665–1671. [CrossRef] [PubMed] [Google Scholar]
- Cisson CM, Wilson BW. 1982. Degenerative changes in skeletal muscle of hens with tri-ortho-cresyl phosphate-induced delayed neurotoxicity: Altered acetylcholinesterase molecular forms and increased plasma creatine phosphokinase activity. Toxicol Appl Pharmacol 64: 289–305. [CrossRef] [PubMed] [Google Scholar]
- Crestani M, Menezes C, Glusczak L, et al. 2006. Effects of clomazone herbicide on hematological and some parameters of protein and carbohydrate metabolism of silver catfish Rhamdia quelen. Ecotoxicol Environ Saf 65: 48–55. [CrossRef] [PubMed] [Google Scholar]
- Ferrando MD, Andreu E. 1991. Effects of lindane on fish carbohydrate metabolism. Ecotoxicol Environ Saf 22: 17–23. [CrossRef] [PubMed] [Google Scholar]
- Fukuhara N, Hoshi M, Mori S. 1977. Core/targetoid fibres and multiple cytoplasmic bodies in organophosphate neuropathy. Acta Neuropathol 40: 137–144. [CrossRef] [PubMed] [Google Scholar]
- Gimeno L, Ferrando MD, Sanchez S, Gimeno LO, Andreu E. 1995. Pesticide effects on eel metabolism. Ecotoxicol Environ Saf 31: 153–157. [CrossRef] [PubMed] [Google Scholar]
- Glusczak L, Miron SD, Crestani M, et al. 2006. Effect of glyphosate herbicide on acetylcholinesterase activity, metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf 65: 237–241. [CrossRef] [PubMed] [Google Scholar]
- Gupta RC, Dettbarn WD, Milatovic D. 2009. Skeletal muscle, In: Handbook of Toxicology of Chemical Warfare Agents, edited by R.C. Gupta. Academic Press, pp. 509–531. [CrossRef] [Google Scholar]
- Gupta RC, Lasher MA, Doss RB, Milatovic D. 2014. Skeletal muscle toxicity biomarkers, In: Biomarkers in Toxicology, edited by R.C. Gupta. Burlington: Elsevier Science, pp. 291–308. [CrossRef] [Google Scholar]
- Hill AJ, Howard CV, Cossins AR. 2002. Efficient embedding technique for preparing small specimens for stereological volume estimation: Zebrafish larvae. J Microsc 206: 179–181. [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. 2005. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86: 6–19. [CrossRef] [PubMed] [Google Scholar]
- Karalliedde L, Senanayake N. 1989. Organophosphorus insecticide poisoning. Br J Anaesth 63: 736–750. [CrossRef] [PubMed] [Google Scholar]
- Kumar R, Nagpure NS, Kushwaha B, Srivastava SK, Lakra WS. 2010. Investigation of the genotoxicity of malathion to freshwater teleost fish Channa punctatus (Bloch) using the micronucleus test and comet assay. Arch Environ Contam Toxicol 58: 123–130. [CrossRef] [PubMed] [Google Scholar]
- Kwon YK, Wee SH, Kim JH. 2004. Pesticide poisoning events in wild birds in Korea from 1998 to 2002. J Wildl Dis 40: 737–740. [CrossRef] [PubMed] [Google Scholar]
- Lukaszewicz-Hussain A. 2010. Role of oxidative stress in organophosphate insecticide toxicity-Short review. Pestic Biochem Physiol 98: 145–150. [CrossRef] [Google Scholar]
- Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB. 2018. Pesticide toxicity: a mechanistic approach. EXCLI J 17: 1101–1136. [PubMed] [Google Scholar]
- Mars M, Gregory MA. 2014. A model for determining baseline morphometrics of skeletal myofibres. J S Afr Vet Assoc 85: 01–06. [CrossRef] [Google Scholar]
- Mahajan R, Blair A, Lynch CF, et al. 2006. Fonofos exposure and cancer incidence in the agricultural health study. Environ Health Perspect 114: 1838–1842. [CrossRef] [PubMed] [Google Scholar]
- Mohamed FA. 2009. Histopathological studies on Tilapia zillii and Solea vulgaris from Lake Qarun, Egypt. World J Fish Mar Sci 1: 29–39. [Google Scholar]
- Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL. 2006. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C Toxicol Pharmacol 143: 141–149. [CrossRef] [PubMed] [Google Scholar]
- Pamanji R, Bethu MS, Yashwanth B, Leelavathi S, Rao JV. 2015. Developmental toxic effects of monocrotophos, an organophosphorous pesticide, on zebrafish (Danio rerio) embryos. Environ Sci Pollut Res 22: 7744–7753. [CrossRef] [PubMed] [Google Scholar]
- Pimentel D, Levitan L. 1986. Pesticides: amounts applied and amounts reaching pests. Bioscience 36: 86–91. [CrossRef] [Google Scholar]
- Pimentel D. 2009. Pesticides and pest control, In: Integrated Pest Management: Innovation-Development Process, edited by R. Peshin, A.K. Dhawan. Dordrecht: Springer, pp. 83–87. [CrossRef] [Google Scholar]
- Pugazhvendan SR, Narendiran NJ, Kumaran RG, Kumaran S, Alagappan KM. 2009. Effect of malathion toxicity in the freshwater fish Ophiocephalus punctatus: a histological and histochemical study. World J Fish Mar Sci 1: 218–224. [Google Scholar]
- Rahman S. 2013. Pesticide consumption and productivity and the potential of IPM in Bangladesh. Sci Total Environ 445: 48–56. [CrossRef] [PubMed] [Google Scholar]
- Richardson M. 1998. Pesticides-friend or Foe? Water Sci Technol 37: 19–25. [CrossRef] [Google Scholar]
- Roex EW, Keijzers R, Van Gestel CA. 2003. Acetylcholinesterase inhibition and increased food consumption rate in the zebrafish, Danio rerio, after chronic exposure to parathion. Aquat Toxicol 64: 451–460. [CrossRef] [PubMed] [Google Scholar]
- Sakr SA, Gabr SA. 1992. Ultrastructural changes induced by diazinon and neopybuthrin in skeletal muscles of Tilapia nilotica. Bull Environ Contam Toxicol 48: 467–473. [PubMed] [Google Scholar]
- Sandoval-Herrera N, Mena F, Espinoza M, Romero A. 2019. Neurotoxicity of organophosphate pesticides could reduce the ability of fish to escape predation under low doses of exposure. Sci Rep 9: 1–11. [PubMed] [Google Scholar]
- Sastry KV, Siddiqui AA. 1984. Some haematological, biochemical and enzymological parameters of a freshwater teleost fish, Channa punctatus, exposed to sublethal concentrations of quinalphos. Pestic Biochem Physiol 22: 8–13. [CrossRef] [Google Scholar]
- Scott GR, Sloman KA. 2004. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68: 369–392. [CrossRef] [PubMed] [Google Scholar]
- Shadnia S, Azizi E, Hosseini R, et al. 2005. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol 24: 439–445. [CrossRef] [PubMed] [Google Scholar]
- Sidhu GK, Singh S, Kumar V, Dhanjal DS, Datta S, Singh J. 2019. Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit Rev Environ Sci 49: 1135–1187. [CrossRef] [Google Scholar]
- Singh RN, Pandey RK, Singh NN, Das VK. 2009. Acute toxicity and behavioral responses of common carp Cyprinus carpio (Linn.) to an organophosphate (Dimethoate). World J Zool 4: 70–75. [Google Scholar]
- U.S. EPA. 2008. Health Effects Support Document For Fonofos. Washington, DC: US Environmental Protection Agency. Available: https://www.epa.gov/sites/production/files/2014-09/documents/health_effects_support_document_for_fonofos.pdf [accessed 16 May 2018] [Google Scholar]
- Üner N, Oruç EÖ, Sevgiler Y, Şahin N, Durmaz H, Usta D. 2006. Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21: 241–245. [CrossRef] [PubMed] [Google Scholar]
- Valenzuela CA, Zuloaga R, Poblete-Morales M, et al. 2017. Fish skeletal muscle tissue is an important focus of immune reactions during pathogen infection. Dev Comp Immunol 73: 1–9. [CrossRef] [PubMed] [Google Scholar]
- Wang G, Xiong D, Wu M, Wang L, Yang J. 2020. Induction of time and dose-dependent oxidative stress of triazophos to brain and liver in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 228: 108640. [CrossRef] [PubMed] [Google Scholar]
- Zhang W, Jiang F, Ou J. 2011. Global pesticide consumption and pollution: with China as a focus. Proc Int Acad Ecol Environ Sci 1: 125–144. [Google Scholar]
Cite this article as: Arman S, İŞisağ Üçüncü S. 2021. Impact of acute fonofos exposure on skeletal muscle of zebrafish: Histopathological and biometric analyses. Ann. Limnol. - Int. J. Lim. 57: 25
All Tables
Measurements of skeletal muscle fiber diameters (μm) of zebrafish following exposure to fonofos for 96 h. Values indicate mean and standard error of measurements. An asterisk (*) indicates significant difference compared to the control (p < 0.001).
All Figures
 |
Fig. 1 Normal histological structure of the skeletal muscle of the control samples. (a) H&E. (b) GT. (c) PAS. F: muscle fiber; n: nucleus; *: glycogen storages; Ellipse: cross-striations. |
| In the text | |
 |
Fig. 2 Skeletal muscle sections of 1 mg/L of fonofos treated group. (a) Fibrosis (fi), H&E. (b) Intramyofibrillar vacuoles (thin arrows) and atrophic fibers (thick arrows), GT. (c) Fibrosis (fi) and disintegrated myofibrils (arrowheads), GT. (d) Decrease in glycogen storage (asterisks), PAS. |
| In the text | |
 |
Fig. 3 Skeletal muscle sections of 2 mg/L of fonofos treated group. (a) Fibrosis (fi) and intramyofibrillar vacuole formation (arrow), H&E. (b) Deformation of general tissue integrity with atrophic myofibers (black arrows) and disappearance of some fibers (white arrows), H&E. (c) Splitting of muscle fibers (double-headed arrow) and disintegrated myofibrils (arrowhead), GT. (d) Progressive decrement in glycogen storage (asterisks), PAS. |
| In the text | |
 |
Fig. 4 Skeletal muscle sections of 4 mg/L of fonofos treated group. (a) Atrophic fiber (arrow), disintegrated myofibrils (arrowheads) and splitting of muscle fibers (double-headed arrow) accompanying with histoarchitectural loss, H&E. (b) Intramyofibrillar vacuoles (arrows), H&E. (c) Intramyofibrillar vacuoles (arrows) and necrosis (N), GT. (d) Severe decrement in glycogen content (asterisks), PAS. |
| In the text | |
 |
Fig. 5 (a) Examples of fiber diameter (d) measurements of the control group (b) alterations in muscle fiber diameter measurements following 96 h fonofos exposed groups compared to the control. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


