| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 53, 2017
|
|
|---|---|---|
| Page(s) | 425 - 465 | |
| DOI | https://doi.org/10.1051/limn/2017022 | |
| Published online | 15 November 2017 | |
Research Article
Diversity and distribution of the Macrothrix paulensis species group (Crustacea: Cladocera: Macrothricidae) in the tropics: what can we learn from the morphological data?
1
A.N. Severtsov Institute of Ecology and Evolution,
Leninsky Prospect 33,
Moscow
119071, Russia
2
Kazan Federal University,
Kremlevskaya Street 18,
Kazan
420000, Russia
* Corresponding author: alexey-a-kotov@yandex.ru
Received:
10
May
2017
Accepted:
4
September
2017
Over the last 20 years significant progress was achieved in morphological investigations of the genus Macrothrix Baird (Cladocera: Macrothricidae). The Macrothrix paulensis species group is known from tropical and subtropical regions all around the World. In this paper we redescribe M. capensis (Sars, 1916) based on material from the Republic of South Africa, and describe a new species, M. australiensis sp. nov. from Australia. A cladistic analysis of 19 morphological characters in 12 taxa (including M. triserialis Brady, 1886 as an outgroup) derived from our analysis of original samples and literature data, resulted in 18 equally-parsimonious trees. Within the M. paulensis group, we can recognize a basal section with five taxa (M. atahualpa Brehm, 1936, M. smirnovi Ciros-Pérez and Elías-Gutiérrez, 1997, M. agsensis Dumont, Silva Briano and Subhash Babu, 2002, M. capensis, M. australiensis sp. nov.) which are both biogeographical and phylogenetic relicts. They occur exactly in well-known zones of cladoceran endemism:: Australia,South Africa, the Andean highlands and Mexican Plateau with surrounded territories. In contrast, the crown group is widely distributed in tropical lowlands. No truly “Pantropical” taxa were found, all taxa could be classified as: (1) exclusively Neotropical; (2) exclusively Australian; (3) Palaeotropical (Afro-Asian); (4) endemics of Mexican Plateau. Probably a combination of scenarios took place during history of the M. paulensis group, but we can conclude that all possible scenarios are old, which confirms antiquity of the M. paulensis group. Australia and Tasmania could be a source of additional species from this group.
Key words: morphology / redescription / new species / pantropical distribution / biogeography / Macrothrix
© EDP Sciences, 2017
1 Introduction
Taxa of the family Macrothricidae Norman and Brady, 1867 (Crustacea: Branchiopoda: Cladocera) are important members of littoral and phytophylous communities in fresh water bodies around the world. Sometimes, especially in the macrophyte zone of tropical water bodies, they occur in a very high abundance (e.g. Thomas, 1961) or, at least, dominate among microscopic animals (Smirnov, 1976; Dumont, 1994). As primary consumers, macrothricids may constitute a significant part of fish diets, including economically important fish species (Baird, 1850; Oliver, 1991; Meschiatti and Arcifa, 2002). However, taxonomy of the Macrothricidae has attracted little attention of cladoceran investigators for a long time. Lack of reliable morphological features for species identification slowed down accumulation of knowledge on the macrothricid diversity and distribution. According to Löffler (1968) macrothricid taxonomy was “hopeless”, and Korovchinsky (1996) even concluded that there were no unambigously accepted (“valid” in his understanding) species among the Macrothricidae at the time of his publication. Smirnov (1976, 1992) performed the first global attempts to accumulate all previous taxonomic data on the family and offered original comprehensive identification keys. Of course, in some cases these keys allowed to identify specimens only to the species group level, but his publications attracted attention of the hydrobiologists to certain macrothricid taxa and became a basis for all subsequent taxonomic works.
Afterwards main efforts were concentrated on the revision of the genus Macrothrix Baird, 1843, where only few investigations concerned a formal establishment of the taxa new to science (Ciros-Pérez et al., 1996; Ciros-Pérez and Elías-Gutiérrez, 1997; Elías-Gutiérrez and Smirnov, 2000). Some papers were dealing with detailed redescriptions of forgotten taxa (Smirnov and Bayly, 1995; Kotov, 1999; Garfias-Espejo et al., 2007; Kotov, 2008) or combined redescriptions of poorly known taxa and descriptions of new species (Silva-Briano et al., 1999; Dumont et al., 2002; Kotov and Hollwedel, 2004; Kotov et al., 2004, 2005; Kotov, 2007b). Moreover, some taxa earlier designated to other genera or subgenera were reinvestigated and transferred to Macrothrix (e.g. Kotov and Hollwedel, 2004; Kotov et al., 2005). As a result of these efforts, data of macrothricid morphology were significantly supplemented according to current standards accepted in cladoceran taxonomy and new diagnostic features were found, making taxon identification more accurate (Silva-Briano, 1998; Dumont and Silva-Briano, 1998; Kotov, 1999, 2008). As a result, a recent understanding of the genus Macrothrix was determined more precisely (Kotov and Hollwedel, 2004; Kotov et al., 2005; Kotov, 2007b, 2008). Although some unresolved problems still remain (especially in discrimination of small-sized taxa), well studied groups within Macrothrix have been outlined to date.
The Macrothrix paulensis species group is among them (Kotov and Hollwedel, 2004; Kotov et al., 2005). This group was first recognized by Kotov and Hollwedel (2004) and later by Kotov et al. (2005), and its relation with dubious genera Iheringula Sars and Echinisca Liévin was discussed. The members of this group inhabit tropical and subtropical water bodies with a developed macrophyte belt. These taxa have a relatively large size (up to 1.5 mm) and peculiar morphological traits: (1) a large, triangular labrum; (2) a subquadrangular postabdomen; (3) large spines at inner margin of antenna I; (4) robust spinules on the seta located on the proximal segment of antenna II endopod; (5) a single ejector hook on the thoracic limb I (see discussion of these features in Kotov et al., 2005). Well-recognized diagnostic features allow us to consider M. paulensis species group as a nice example both for detailed morphological comparison and biogeographical speculations.
According to Kotov and Hollwedel (2004) and Kotov et al. (2005), the M. paulensis group include three well described species in tropical regions of the New World (M. paulensis (Sars, 1900), M. sioli (Smirnov, 1982) and M. brandorffi Kotov and Hollwedel, 2004) and two species from the Old World (M. odiosa Gurney, 1916 and M. pholpunthini Kotov, Maiphae and Sanoamuang, 2005). M. malaysiensis Idris and Fernando, 1981 from Malaysia (Idris and Fernando, 1981b) is considered as a closest relative of this group, but it still has not been investigated in detail due to its rarity (Kotov et al., 2005). M. atahualpa Brehm, 1936 inhabits the Andes and is also considered as a relative of the M. paulensis group, but differing from the latter in the morphology of the ventral margin of the head and armature of antenna I (Kotov et al., 2010). Remarkably, material of the paulensis-group from Africa and Australia was not studied in detail during previous revisions. Only Smirnov (1992) tried previously to investigate populations of the paulensis-like macrothricids from these two continents, yet he did not reveal any valuable peculiarities of African and Australian populations.
This situation reflects the main pattern of recently conducted taxonomic revisions. Tropical regions of the New World are intensively investigated by methods of classical morphological (Cervantes-Martínez et al., 2000; Sinev and Hollwedel, 2002; Sinev et al., 2004; Kotov et al., 2005; Dumont et al., 2013; Elmoor-Loureiro, 2014; Sousa et al., 2015, Sousa et al., 2016a, b and others) and molecular analysis (Elías-Gutiérrez and Valdez-Moreno, 2008; Elías-Gutiérrez et al., 2008). Also, taxonomic papers of high quality were published on the Cladocera of South Asia (e.g. Padhye and Dumont, 2014; Neretina and Sinev, 2016) and Southeast Asia (Sinev and Sanoamuang, 2007; Kotov et al., 2013b; Van Damme and Maiphae, 2013; Van Damme et al., 2013a; Sinev et al., 2016 and others). At the same time, detailed taxonomic publications dealing with African cladocerans are not so numerous (Sinev, 2006, 2008; Van Damme and Dumont, 2009; Kotov and Taylor, 2010; Van Damme et al., 2013b; Neretina and Kotov, 2015; Neretina and Sinev, 2016). Also many Australian taxa are still waiting for a reassessment on the current level of morphological analysis (e.g. Smirnov, 1995), although some detailed taxonomic works concerning Australia were published as well (Sinev, 1997, 2004; Van Damme et al., 2007; Sinev and Shiel, 2012).
Keeping in mind obvious problems concerning the cladoceran taxonomy in Africa and Australia, we were not surprised when we found a forgotten species of the paulensis-group, M. capensis (Sars, 1916), in the Republic of South Africa and discovered populations belonging to a similar form turning put to be a new taxon from Australia. Moreover, our re-consideration of some taxa from Mexican plateau earlier regarded as members of M. triserialis group (Dumont et al., 2002) led to conclusion that they are, in reality, also members of the M. paulensis group.
The main aims of our paper are: (1) to redescribe morphology of M. capensis in detail; and (2) to describe a new species of the paulensis-group from Australia; (3) to discuss the morphology of M. odiosa with clarification of some ambiguities in its taxonomy in Africa; (4) to compare the morphology of all currently well-described nowadays members of the paulensis-group; (5) to analyze original and literature data on their distribution and to evaluate potential zoogeographic scenarios.
2 Materials and methods
Samples in 4% formaldehyde from Africa (the Republic of South Africa, Namibia and Ethiopia), Southeast Asia (Thailand), Australia and South America (Chile) were preliminarily inspected in small Petri dishes under a stereoscopic binocular microscope LOMO. Specimens were transferred to drops of glycerol–formaldehyde mixture on slides and examined under an Olympus BX41 light microscope. At least two adult parthenogenetic females and two adult males (where they were available) from each sample were dissected via tungsten needles, and features important for the taxon identification were checked.
Several specimens were dehydrated in increasing ethanol series (30, 50, 70, and twice in 96%), transferred to 100% acetone (40 min each series), and to hexamethyldisilazane (40 min) (Laforsch and Tollrian, 2000). Then specimens were dried overnight on air, covered with gold via S150A Sputter Coater (Edwards, UK) and investigated under scanning electron microscope CamScan MV 2300 (Tescan, Czech Republic), at accelerating voltage 20 kV and working distance 15 mm.
For morphological descriptions we used terminology summarized by Kotov (2013).
A cladistic analysis was performed using PAUP program Vers. 4.0a for 32 bit Microsoft Windows (Swofford, 1993), using branch-and-bond search with an aim to elucidate the possible phylogeny of M. paulensis group. We considered M. triserialis group is an outgroup to the M. paulensis-like species. In some cases characters used in this analysis vary within the latter and were encoded as “data missing”. A bootstrap simulation of 1000 replications was performed as a test of the robustness of these analyses.
Abbreviations for collections. AAK, Personal collection of A.A. Kotov, A.N. Severtsov Institute of Ecology and Evolution (Moscow, Russia). ANN, Personal collection of A.N. Neretina, A.N. Severtsov Institute of Ecology and Evolution (Moscow, Russia). MGU, Collection of the Zoological Museum of M.V. Lomonosov Moscow State University (Moscow, Russia). NNS, Personal collection of Prof. N.N. Smirnov, A.N. Severtsov Institute of Ecology and Evolution (Moscow, Russia). SAM, South Australian Museum (Adelaide, Australia).
Abbreviations in illustrations and text. I–V = thoracic limbs I–V; e1–e5 = endites 1–5 of thoracic limbs; ejh = ejector hook on limb I; epp = epipodite; ext = exopodite; IDL = inner distal lobe of limb I; ODL = outer distal lobe of limb I; pep = preepipodite; s = sensillum.
3 Results
(1) Redescription of Macrothrix capensis (Sars, 1916) versus M. odiosa Gurney, 1916 and questions on their distribution within Africa
Order Anomopoda Sars, 1865
Family Macrothricidae Norman and Brady, 1867
Genus Macrothrix Baird, 1843
Macrothrix capensis (Sars, 1916)
Sars (1916): p. 323–324, plate XXXVI, figs. 1a–d.
Smirnov (1992): p. 87–89, figs. 363–374.
Type material. Apparently lost.
Type locality. Port Elizabeth, Eastern Cape, the Republic of South Africa.
Material examined (all samples from the Republic of South Africa). Over 10 parthenogenetic females from Eastern Cape, collection details are unknown, NNS-1997-054; 10 parthenogenetic females from Kruispad (S 32.8700°, E 18.2564°), Western Cape, coll. 10.08.2000 by G. Jones, NNS-2002-205; 10 parthenogenetic females from Springfield (S 34.7375°, E 19.9111°), Western Cape, coll. 24.08.2000 by G. Jones, NNS-2002-215; 10 parthenogenetic females from Wiesdrif (S 34.6750°, E 19.9041°), Western Cape, coll. 23.09.2000 by G. Jones, NNS-2002-219; 10 parthenogenetic females from Lang Pan (S 34.6161°, E 19.8917°), Western Cape, coll. 23.08.2000 by G. Jones, NNS-2002-225; 1 parthenogenetic female from Langvlei (S 33.9914°, E 22.6947°), Western Cape, coll. 11.10.2000 by G. Jones, NNS-2002-236; 10 parthenogenetic females from Langvlei (S 33.9914°, E 22.6947°), Western Cape, coll. 11.10.2000 by G. Jones, NNS-2002-237; 5 parthenogenetic females from Grootrondevlei (S 34.2383°, E 18.3825°), Western Cape, coll. 25.10.2000 by G. Jones, NNS-2002-239; 15 parthenogenetic females from Skulpadvlei (S 34.3275°, E 18.4508°), Western Cape, coll. 06.10.2000 by G. Jones, NNS-2002-240; 10 parthenogenetic females and two ephippial females from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244; 15 parthenogenetic females from Soetendalsvlei wetland (S 34.3672°, E 18.8847°), Western Cape, coll. 31.01.2001 by G. Jones, NNS-2002-246; 3 parthenogenetic females from an unknown locality, coll. in September of 1997 by M. Groodman, NNS-2002-262.
Diagnosis. Species of large size for the genus (length of adult parthenogenetic female up to 1.3 mm). Body of parthenogenetic female as for genus (see Smirnov, 1992). Dorsum not elevated significantly above head. Serration on dorsum not expressed. Posterodorsal angle of valves smooth. Head pore located on the level of head. Ventral head margin with a projection. Labrum of moderate size, rounded. Postabdomen subquadrangular, postabdominal flaps not prominent. Anal margin of postabdomen covered by fine denticles. Antenna I rod-like, with a row of bunches of gracile denticles. Antenna II as for the genus. Armature of proximal endopod segment seta of antenna II represented by a row of robust denticles. Spine on the second exopod segment seta of antenna II long, reaches 1/2 length of third exopod segment. Thoracic limb I bears a single ejector hook. Thoracic limb II as for the genus. On exopodite of thoracic limb III seta 3 subequal in length to seta 2. On exopodite of thoracic limb IV seta 2 almost subequal in size to seta 1. Thoracic limb V as for the genus. Ephippial female similar to parthenogenetic female. Ephippium typical for macrothricids, brownish, with elongated inflated hillocks, containing two eggs. Male as for the genus, male seta in the middle of antennular body.
Redescription
Parthenogenetic female. In lateral view body ovoid (Fig. 1A), maximum height at middle of body (body height/length ratio about 0.65). In dorsal and ventral view body compressed laterally. Dorsal margin arched from tip of rostrum to posterior most point, interrupted by a low dome over compound eye, and small depression posterior to dorsal head pore (Fig. 1A). Dorsal margin of valves not elevated significantly above dorsal margin of head (Fig. 1A). Posterodorsal margin broadly rounded (Fig. 1A). Posterodorsal angle smooth, obtuse (Fig. 1A). Ventral margin convex, covered by setae of different size in different regions of valves (Fig. 1A, D–F). Anteroventral angle rounded (Fig. 1A). Valves with a sculpture represented by polygons (Fig. 3C).
Head large (Fig. 1B), head length from tip of rostrum to border with valves makes up to 0.38 times of body length. In lateral view, dorsal margin of head with a low dome above eye (Fig. 1B). Head ventral margin with a single rounded projection (Figs. 1B, 3A). Compound eye significantly larger than ocellus (Fig. 1B). In anterior view, rostrum compressed laterally, with a small split-like frontal head pore located close to its frontal edge. Dorsal head pore large, rounded, with prominent ring around it, located on posterior part of head shield (Fig. 3B). Labrum of moderate size, with rounded apical portion (Fig. 1C). Ventral margin of labral keel with four transverse rows of setules (Fig. 1C). Distal labral appendage finely setulated (Fig. 1C).
Thorax relatively long (Fig. 1A). Abdomen short (Fig. 1A).
Postabdomen subrectangular in lateral view (Figs. 1G, 3D), slightly narrowing distally; postabdomen length/height ratio about 3.3. Ventral margin straight to slightly convex, with a bunch of fine setules (Figs. 1G, 3D). Preanal margin long, in about 2.6 times longer than anal margin. Postanal margin in three times shorter than anal margin (Figs. 1G–H, 3D–E). Preanal margin bears bunches of stiff setules. Also, transverse rows of stiff setules covered postanal and anal margins, but there are no hair-like setules (Figs. 1H, 3E) (in contrast to M. paulensis and M. brandorffi, see Kotov and Hollwedel (2004)). Not prominent postabdominal flaps at side of anus (Fig. 1G–H). Postabdominal seta as long as postabdomen (Figs. 1G, 3D), with a short distal segment. Unfortunately, due to failed fixation, setules on postabdominal seta were not kept. Postabdominal claw small (smaller than postanal margin of postabdomen), curved, with a pointed tip and a relatively wide base in lateral view (Figs. 1H–J, 3E). There are several denticles on its dorsal side and more fine denticles on ventral side (Figs. 1I, 3E). Inner part of claw covered by undulated row of small denticles (Fig. 1J).
Antenna I “rod-like” in terminology of Smirnov (1992), long and almost straight, not dilating to apex (Figs. 2A, 3A, F–G). Bunches of 2–3 long slender denticles at inner margin of antenna I (Figs. 2A, 3G). Whole surface of antenna I covered by transverse rows of spinules. Distal edge bears long slender denticles (Figs. 2A, 3G). Antennular sensory seta slender, arising from outer side of proximal part (Fig. 2A). Nine aesthetascs, two of them longer and thicker than the rest (Fig. 2A). Each thicker aesthetasc bears two minute “claws” at the apex (Fig. 2A).
Antenna II large (Figs. 1A, 2B, 3H), coxal region folded, with two small sensory setae unequal in size. Antennal formula: setae 0-0-1-3/1-1-3, spines 0-1-0-1/0-0-1. Basal segment robust, covered by transverse rows of fine spinules (Figs. 1A, 2B, 3H). Small spine located on outer surface of basal segment, a bisegmented short sensory seta on inner surface, it almost reaches third exopod segment. Exopod and endopod subequal in size (Figs. 1A, 2B, 3H–I). All their segments cylindrical, elongated, covered by transverse rows of fine spinules (Fig. 3H–I). Apical swimming setae long, subequal in length, bearing fine spinules and long setules (Figs. 1A, 2B, 5D). Lateral seta of basal endopod segment (Fig. 2B–C) larger than other setae and armed with two rows of spinules: spinules on the edge of this seta thin and densely located (distance between two neighboring spinules is almost equal to width of seta), spinules on the outer surface of this seta more robust and sparsely located (distance between two neighboring spinules significantly – commonly in 3.5 times – larger than width of seta) (Figs. 2D–E, 5A). Seta on middle endopod segment reaches tips of apical setae, covered by long setules and fine spinules (Fig. 2B). Lateral seta of third exopod segment has the same armature (Figs. 2B, 5C). True spine on second exopod segment thin, reaches 1/2 length of third exopod segment (Figs. 2B, 3H–I). Second and third exopod segment bear short additional spines (we do not represent them in the antennal formula) (Figs. 2B, 3H–I). Normally, these additional spines almost subequal in size and three times shorter than true spine on second exopod segment. Spines of both apical exopod and endopod segments thin, exopod apical spine in two times longer than endopod spine (Figs. 2B, 3H–I, 5C).
Thoracic limbs: five pairs (Fig. 4A–G).
Limb I large (Fig. 4A–B). Epipodite ovoid, with a long finger-like projection (Fig. 4A). Accessory seta small (Fig. 4B). ODL conical and large, bearing a single long bisegmented seta, its distal segment feathered unilaterally (Fig. 4B). IDL massive, covered by rows of stiff setules, with three bisegmented setae of different size, each unilaterally setulated in distal part (Fig. 4B). Limb corm almost rectangular in lateral view (Fig. 4A). Endite 4 with three posterior soft setae (among them seta a the longest, with long fine setules on its distal segment, setae b and c shorter, subequal in length, covered by fine setules in proximal part and stiff short setules in distal part) and a single stiff anterior seta 1 (Fig. 4A). Endite 3 with three soft posterior setae unequal in size (among them seta d the longest, bearing fine setules both in proximal and distal segments, seta e and f armed unilaterally by rough setules in their distal portions) and a single fork-like anterior seta 2 (Fig. 4A). Endite 2 with two posterior bisegmented setae subequal in size, covered by fine short setules, and a single anterior fork (Fig. 4A). Endite 1 with two bisegmented soft setae. A single ejector hook with setulated distal segment (Fig. 4A).
Limb II triangular-rounded (Fig. 4C). Exopodite ovoid, covered by rows of fine setules, and bearing a single long soft seta. Inner portion of limb II with eight scrapers, among them setae 1 and 2 the longest, setae 3–5 somewhat shorter, and setae 6–8 short (Fig. 4C). Setae 1 and 2 unilaterally covered by stiff fine denticles in their distal portion. Seta 3 bears fine spinules; setae 4-8 feathered by more robust denticles. A deep incision between endite 2 and endite 1. Portion of gnathobase (= endite 1) bordering endite 2 somewhat inflated and bears a row of fine setules. Distal armature of gnathobase with four elements. Filter plate with four bisegmented setae, subequal in length (Fig. 4C).
Limb III (Fig. 4D–E) with subrectangular exopodite (Fig. 4D), bearing a single lateral seta and three distal setae (among them, the middle seta somewhat longer than others). Distal endite in terms of Kotov (2013) with three anterior setae (seta 1 covered by small denticles, setae 2 and 3 bear fine setules on their distal portions), small sensillae near seta 2 and seta 3 (Fig. 4D–E). Proximal endite with a small elongated sensillum and three setae (compare with M. elegans Sars, 1901, which has a small bottle-shaped sensillum and four setae on proximal endite (Kotov et al., 2004)) (Fig. 4E). Six setae on posterior face of limb (a–f) (among them seta a short and thick, with stiff setules on its distal portion and long setules on proximal portion; other setae increasing in size proximally). Distal armature of gnathobase with four elements, one of them a bottle-shaped sensillum (Fig. 4E). Filter plate absent (Fig. 4D).
Limb IV (Fig. 4F) with relatively small rounded exopodite, bearing distally two soft setae, subequal in size. Inner distal portion with four anterior setae (1–4) (among them seta 1 covered by short stiff setules, setae 2–4 bearing more long setules) and small sensillum near each seta 2 and seta 3 (Fig. 4F). Posterior face with five soft setae (a–e) increasing in size proximally (Fig. 4F). Distal armature of gnathobase consists of four elements: a small bottle-shaped sensillum, bisegmented seta and two elongated projections. Filter plate absent (Fig. 4F).
Limb V (Fig. 4G) with a three-lobed preepipodite covered by fine setules. Epipodite relatively large, ovoid (Fig. 4G). Exopodite with a single seta. Inner distal portion as small flap, covered by setules; three setae on its inner margin (the distalmost seta significantly longer than others) (Fig. 4G). Filter plate absent (Fig. 4G).
Ephippial female. In lateral view, body proportions as in parthenogenetic female. A chitinized plate along dorsal margin on body (Fig. 5B). Almost all valves area is included to the constitution of the ephippium. Surface of ephippium brownish, with elongated inflated hillocks, boundaries between exuviated and unexuviated parts are not delineated (Fig. 5B, E). Two eggs in ephippium.
Males. Not found in our material, but Sars (1916) published a detailed description and a realistic illustration for male of M. capensis (Sars, 1916: p. 324, plate 36: figs. 1d). According to his description, male body subrectangular, dorsum almost straight, posterodorsal angle rounded. Sensory seta and male seta on antenna I located quite far from each other.
Size. Maximum length of adult parthenogenetic females 1.3 mm, height 0.84 mm. Maximum length of ephippial females 0.75 mm, height 0.51 mm. Male size unknown, Sars (1916: p. 324) only stated that the male is scarcely half as large as female.
Variability. No significant variability between investigated individuals from all South African localities was found.
Distribution. According to Smirnov (2008) M. capensis is a common taxon in the Republic of South Africa. Based on our original data, M. capensis is known only from South Africa. Most populations are found in Western Cape, although originally this species was described from Eastern Cape (Sars, 1916), but some populations are present in other parts of the southern half of the Republic of South Africa, e.g. in Drakensberg mountains.
Differential diagnosis. There are only two taxa from the paulensis-group in Africa, M. capensis and M. odiosa, which are different in fine morphological traits of both females and males (Tab. 1). The main difference between M. capensis and M. atahualpa concerns some fine details: proportions of distal segment of postabdominal seta (this segment is short in M. capensis and relatively long in M. atahualpa), structure of thoracic limb II (additional soft seta between scraper 4 and scraper 5 is absent in M. capensis and present in M. atahualpa). See differential diagnosis of M. australiensis sp. nov. for differences from the latter.
Macrothrix odiosa Gurney, 1916
Macrothrix tenuicornis Gurney, 1907: p. 25, plate 1: figs. 1–2, plate 2: fig. 22 – junior homonym of M. tenuicornis Kurz, 1875: p. 32–34, pl. 3: fig. 1.
Macrothrix odiosa Gurney, 1916: p. 335; Behning (1938: p. 294, fig. 2; 1941: 225–227, fig. 97); Brehm (1952: p. 41–42, figs. 4–5); Manujlova (1964: p. 185–186, fig. 80); Mukhamediev (1986: p. 69–73, fig. 17); Smirnov (1992: 89–93, figs. 375–393); Saeng-aroon (2001: p. 36: fig. 19); Saeng-aroon and Sanoamuang (2002: p. 16, fig. 8).
Macrothrix capensis var. monodi Gauthier, 1930: p. 95–98, figs. 2a–c.
Macrothrix capensis monodi Gauthier, 1930 in Idris and Fernando (1981a: p. 238–239, figs. 11–15); Idris (1983: p. 47, fig. 22).
Macrothrix madagascariensis (Brehm, 1930) in Brehm (1933: p. 691) and Brehm, (1952: p. 41).
Macrothrix monodi Gauthier, 1930 in Dumont and Van de Velde (1977: p. 85–87, figs. 5a–f).
Macrothrix orbicularis Brehm, 1930 in Brehm (1930: p. 681–686, figs. 4–6).
Macrothrixcf. paulensis (Sars, 1900) in Sanoamuang, 1998: p. 48, figs. 15–20.
Echinisca odiosa (Gurney, 1916) in Smirnov (1976: p. 118–119, figs. 94–95); Fernando (1980: tab. 1); Ibrasheva and Smirnova (1983: 66–67: fig. 16); Fernando and Kanduru (1984: p. 72: tab. 1); Michael and Sharma (1988: p. 111–113, figs. 35a–c).
Echinisca capensis monodi (Gauthier, 1930) in Smirnov (1976: p. 122, 124).
Echinisca madagascariensis (Brehm, 1930) in Smirnov (1976: p. 130).
Echinisca orbicularis (Brehm, 1930) in Smirnov (1976: p. 130).
(?) Echinisca sumatrensis (Brehm, 1933) in Smirnov (1976: p. 130).
Gurneyella madagascariensis Brehm, 1930 in Brehm (1930: p. 681–686, figs. 4–6).
Gurneyella monodi (Gauthier, 1930) in Brehm (1934: p. 59–61, figs. 4–6); Rey and Saint-Jean (1969: p. 29–31, figs. 8a–d).
Gurneyella odiosa (Gurney, 1916) in Biswas (1971: p. 127, figs. 7g–i).
(?) Gurneyella sumatrensis Brehm, 1933 in Brehm (1933: p. 692–693, figs. 17–20); Rammner (1937: p. 44–45, figs. 6–9).
Material examined here from Southeast Asia (Fig. 6): 2 parthenogenetic females from Lake Kud-Thing in floodplain of Mekong River, Nong Khai Province, Thailand, coll. 28.11.1998 by C. Saeng-aroon, AAK-2003-033; 2 parthenogenetic females from Lake Kud-Thing in floodplain of Mekong River, Nong Khai Province, Thailand, AAK-2004-049; 1 parthenogenetic female from Vientiane Province, Laos, coll. by S. Siboualipha, AAK-2012-043.
Material examined here from Africa (Figs. 7–19): 10 parthenogenetic females from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001; 5 parthenogenetic females from a pool, “Zara” between Hamusit and Worota (N 11.8166°, E 37.6000°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-002; 5 parthenogenetic females from a pool, “Gosho” near to junction of D'tabor and Adiss Zemen road (N 11.9500°, E 37.7000°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-003; 3 parthenogenetic females from Lake Liambezi (S 17.9166°, E 24.3333°), Zambezi floodplain, E. Caprivi, the Republic of Namibia, coll. 10.12.1982, AAK-1998-083; 2 parthenogenetic females from Crane Pan 5, Cobham (S 29.6658°, E 29.3717°), KwaZulu-Natal, the Republic of South Africa, coll. 15.02.1998 by K. Martens and Hamer, NNS-2002-009; 10 parthenogenetic females from Crane Tarn 3, Cobham (S 29.7122°, E 29.3219°), KwaZulu-Natal, the Republic of South Africa, coll. 16.02.1998 by K. Martens and Hamer, NNS-2002-010; 3 parthenogenetic females from Loteni (S 29.3772°, E 29.5425°), KwaZulu-Natal, the Republic of South Africa, coll. 18.03.1996 by K. Martens and Hamer, NNS-2002-020; 2 parthenogenetic females from rock pool 1 (S 29.6739°, E 29.3303°), Cobham, KwaZulu-Natal, the Republic of South Africa, coll. 11.11.1996 by K. Martens and Hamer, NNS-2002-044; 3 parthenogenetic females from Crane Tarn 1 (S 29.7133°, E 29.3238°), Mzimkhulwana, KwaZulu-Natal, the Republic of South Africa, coll. 21.03.1995 by K. Martens and Hamer, NNS-2002-058; 3 parthenogenetic females from Crane Tarn 2 (S 29.7125°, E 29.3219°), Mzimkhulwana, KwaZulu-Natal, the Republic of South Africa, coll. 21.03.1995 by K. Martens and Hamer, NNS-2002-059; 5 parthenogenetic females from Sentinels Plateau Pool 4, Cobham (S 29.6333°, E 29.3939°), KwaZulu-Natal, the Republic of South Africa, coll. 22.03.1995 by K. Martens and Hamer, NNS-2002-066; 4 parthenogenetic females from Siphongweni tarn 2 (S 29.6833°, E 29.3553°), Cobham, KwaZulu-Natal, the Republic of South Africa, coll. 25.03.1995 by K. Martens and Hamer, NNS-2002-077; 5 parthenogenetic females from Sugarloaf Tarn (S 29.2433°, E 29.5106°), Giant Castle, KwaZulu-Natal, the Republic of South Africa, coll. 27.03.1995 by K. Martens and Hamer, NNS-2002-084; 1 parthenogenetic female from Mbaneni rain pool (S 27.6242°, E 32.2575°), Mkuzi Game Reserve, KwaZulu-Natal, the Republic of South Africa, coll. 28.10.1994 by K. Martens, Hamer and Coke, NNS-2002-109; 2 parthenogenetic female from Pan/Dam on Pot River tributary (S 30.9908°, E 28.2647°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-126; 1 parthenogenetic female from Rush Valley Pan (S 30.8506°, E 28.2156°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03. 1993 by K. Martens, de Moor and Barber, NNS-2002-127; 6 parthenogenetic females from Glen Avis pool 2 (S 30.8061°, E 28.2117°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-129; 15 parthenogenetic females from Glen Avis pool 3 (S 30.8072°, E 28.2161°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-130; 8 parthenogenetic females, 1 male and 2 ephippial females from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131; 1 parthenogenetic female from Pan 1 Rd Middelburg-Hofmeyer (S 31.6917°, E 25.4931°), Karoo, Eastern Cape, the Republic of South Africa, coll. 07.04.1993 by K. Martens, NNS-2002-148.
Comments on parthenogenetic female. Morphology of parthenogenetic and ephippial females was identical in all studied African and Asian populations (see complete morphological description for Asian populations of M. odiosa in Kotov et al. (2005) and our Figs. 6–17, 18A–C). There is just one small-scale feature, which we would like to clarify: the projection on the ventral head margin is not double (see also fig. 24 in Kotov et al., 2005) and looks like as an ellipsoid in the ventral view (Fig. 13C).
Adult male (Figs. 18D–E, 19). Body ovoid, dorsal margin interrupted by a shallow depression between head and valves (Figs. 18D, 19A). Dorsal margin of valves almost straight and not elevated above head (Fig. 19A). Posterodorsal angle distinct, without spine (Fig. 19A). Posteroventral region of valve margin widely rounded; ventral margin significantly convex (Fig. 19A). Head large, with prominent supraocular dome (Figs. 18E, 19B). Ventral margin of head convex, but without prominent projection, typical for parthenogenetic female (Fig. 19B).
Postabdomen subquadrangular (Fig. 19C) (in contrast to adult male of M. capensis having a distal part of postabdomen sub-conical (Sars, 1916: plate 36, fig. 1d)), similar in general shape and armature with that in female. Postabdominal flaps prominent (Fig. 19C). Gonopores open on ventral sides of postabdomen near claw base (Fig. 19C).
Antenna I (Fig. 19D) almost subequal in length to head length, rod-like, its inner margin bears robust long denticles and small spinules. Male seta located on the field with fine long setules, and subequal in size to sensory seta. Male and sensory setae located on same level (almost near antenna base), but on opposite sides of antenna I body (Fig. 19D). Nine terminal aesthetascs, two of them significantly longer than others (Fig. 19D). Proximal endopod segment seta of antenna II with a row of robust denticles alternating with stiff setules and similar with that in female (Fig. 19A).
Thoracic limb I (Fig. 19E–F) with ODL and IDL as in female (male seta not found) (Fig. 19E), copulatory hook slightly curved, its distal portion covered by fine spinules (Fig. 19F).
Size. A single investigated male 0.41 mm in length and 0.24 mm in height.
Variability. A single male was found in a single sample from the Republic of South Africa, just its complete description is represented here.
Distribution around the world. Widely distributed in tropical-subtropical regions of the Old World, see Kotov et al. (2005).
Taxonomic comments. Above we reproduced a list of synonyms, proposed by Kotov et al. (2005), and subsequently added with: (1) M. capensis var. monodi (from Gauthier, 1930), (2) G. monodi (from Brehm, 1934 and Rey and Saint-Jean, 1969), (3) M. madagascariensis (from Brehm, 1930, 1933, 1952) (= G. madagascariensis), (4) M. orbicularis (from Brehm, 1930) and (5) M. monodi (from Dumont and Van de Velde, 1977). Also, we listed the synonyms associated with the generic name Echinisca for African specimens from Smirnov (1976).
Gauthier (1930: p. 92) described M. capensis var. monodi from Silet (Algeria, North Africa). He apparently dealt with a member of the M. paulensis group. Some diagnostic features were illustrated in his figures: a rounded projection on ventral head margin (fig. 2b) and a subquadrangular postabdomen (fig. 2c). At the same time, armature of antenna I was not described in detail, and, obviously, armature of the large seta located on proximal endopod segment is represented inadequately (fig. 2a). We have no opportunities to reexamine the type material, because Gauthier's collection was nationalized by the Algerian government and there is no information about the place, where the collection is now (Hudec, 1993). Based on our data, the distribution range of M. capensis is only restricted exclusively by South Africa. Examined populations from Ethiopia and the Republic of Namibia belong to M. odiosa, moreover this taxon penetrates in the eastern part of South Africa, but it was not found by us in Western Cape, one of the most important centers of cladoceran endemism within South Africa (Van Damme et al., 2013b). Therefore M. odiosa is a widely distributed species of the M. paulensis group in Africa, and we offer to consider M. capensis var. monodi as a junior synonym of M. odiosa. The same idea was proposed by Kořínek (1984).
African specimens investigated by Brehm (1934), Rey and Saint-Jean (1969), Dumont and Van de Velde (1977) apparently belong to M. odiosa due to their subquadrangular postabdomen with prominent anal flaps and robust large denticles on the inner side of antenna I (see in Brehm, 1934: figs. 5a–b, 6; Rey and Saint-Jean, 1969: figs. 8b, d; Dumont and Van de Velde, 1977: figs. 5b, f). It enables us to consider G. monodi and M. monodi, the names used in these publications, as a junior synonyms of M. odiosa as well.
A particular difficult task is to clarify the status for two taxa described from Madagascar by Brehm: M. madagascariensis (Brehm, 1930) (= G. madagascariensis) and M. orbicularis Brehm, 1930. Brehm's type material is apparently lost. Descriptions and drawings of M. orbicularis do not contain important diagnostic features (Brehm, 1930: p. 681–686, figs. 4–6), and a single helpful trait is a subquadrangular postabdomen in both these taxa (Brehm, 1930: fig. 6). Descriptions for M. madagascariensis are dubious and very incomplete (Brehm, 1930: p. 682–683, figs. 3a–b) (see also in Brehm, 1933: p. 691 and Brehm, 1952: p. 41). Brehm himself listed the name M. odiosa for populations from Madagascar as well. Unstable generic position (there were at least three generic names for large-bodied African Macrothrix species: Echinisca Liévin, Gurneyella Brehm and Macrothrix Baird) had brought additional confusion to the taxonomy of this group. Kořínek (1984) and Smirnov (1992) considered M. madagascariensis and M. orbicularis as junior synonyms to M. odiosa. We also expect that Madagascar is inhabited by M. odiosa. This is the most common African species from the M. paulensis group. It is known that usually the cladoceran faunas of tropical islands do not contain a large number of endemic taxa (Schabetsberger et al., 2009; Van Damme, 2016). For Madagascar no endemic cladocerans are known to date based on a current level of revision of the cladocerans from this island (see e.g. Schabetsberger et al., 2013; Neretina and Sinev, 2016). K. Van Damme (personal communication) revealed few chydorid endemics in the samples from Madagascar, but these data are still unpublished.
Thus, in Africa, M. odiosa was found in Ethiopia (our data), Zambia (Kořínek, 1984: p. 51–52, plate 28: figs. 1–9), Chad (Rey and Saint-Jean, 1969); Nigeria (Dumont and Van de Velde, 1977), the Republic of Namibia (our data), Madagascar (Brehm, 1952: p. 41), the Republic of South Africa (our data). It is common in tropical and subtropical Asia (see Kotov et al., 2005). Therefore M. odiosa is a widespread taxon from the M. paulensis group in tropical regions of the Old World, while the distribution range of M. capensis is only restricted to South Africa. These two species clearly differ from each other in morphological features of both females and males (see Tab. 1) and can be hardly confused. The large list of synonyms for M. odiosa, from the one hand, reflects the really vast distribution range of M. odiosa in the Old World, and, from the other hand, admiration by previous authors of the beauty of this peculiar large macrothricid.
(2) Description of new species of the M. paulensis-group from Australia
Macrothrix australiensis sp. nov.
(?) Echinisca capensis capensis (Sars, 1916) in Smirnov and Timms (1983): p. 76, fig. 89a–d.
(?) Echinisca capensis capensis (Sars, 1916) in Smirnov (1976): p. 122, fig. 101.
Etymology. This new species is named after Australia, the continent where it was discovered. This name is intended to reflect a continental endemism, one of the main peculiarities in cladoceran distribution.
Type locality. Lake Fox (37.166°S, 139.777°E), South Australia.
Type material.
Holotype: an adult parthenogenetic female preserved in 96% ethanol and deposited to the collection of South Australian Museum, SAMA C11721. The label of holotype is: “Macrothrix australiensis sp. nov., 1 parth. ♀ from Lake Fox, HOLOTYPE”.
Allotype: an adult male preserved in 96% ethanol and deposited to the collection of South Australian Museum, SAMA C11722. The label of allotype is: “Macrothrix australiensis sp. nov., 1 ♂ from Lake Fox, ALLOTYPE”.
Paratypes: 20 undissected parthenogenetic females preserved in 96% ethanol and deposited to the collection of South Australian Museum, SAMA C11723 and SAMA C11724; 10 undissected parthenogenetic females preserved in 96% ethanol and deposited to the collection of the collection of Zoological Museum of M.V. Lomonosov Moscow State University: MGU Ml 160.
Other material studied. 10 parthenogenetic females from Lake Fox (individuals from laboratory culture of A.V. Makrushin), South Australia, collection details unknown, AAK-1998-053; 3 parthenogenetic females from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-1998-054; 10 parthenogenetic females, 2 ephippial females and 3 males from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197; 5 parthenogenetic females from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, NNS-1997-071; 10 parthenogenetic females, 1 ephippial female and 1 male from Lake Ada, region of Kingscote, Kangaroo Island (S 35.9167°; E 137.3667°), South Australia, coll. 09.01.1976 by B.V. Timms, NNS-1997-168.
Diagnosis. Species of large size for the genus (length of adult parthenogenetic female up to 1.00 mm). Dorsum not elevated significantly above head. Serration on dorsum not expressed. Posterodorsal angle of body smooth. Head pore located on the level of head. Ventral head margin with a projection. Labrum of moderate length, with a rounded apical portion. Postabdomen subquadrangular, postabdominal flaps not prominent. Anal margin of postabdomen covered by fine denticles. Antenna I rod-like, with a row of bunches of gracile short denticles. Armature of proximal endopod segment seta of antenna II represented by a row of robust denticles. Spine on the second exopod segment seta of antenna II short, reaches 1/3 length of third exopod segment. Thoracic limb I bears a single ejector hook. On exopodite of thoracic limb III seta 3 subequal in length to seta 2. On exopodite of thoracic limb IV seta 2 almost subequal in size to seta 1. Ephippial female similar with parthenogenetic female. Ephippium typical for macrothricids, brownish, containing two eggs. Male as for the genus, male seta located in the middle of antennular body.
Description
Parthenogenetic female. In lateral view, body ovoid, maximum height at middle of body (body height/length ratio about 0.67) (Fig. 20A). In dorsal and ventral view body compressed laterally. Dorsal margin arched from tip of rostrum to posterior most point, not interrupted over compound eye (Fig. 20A). Dorsal margin of valves almost straight or concave not elevated above dorsal margin of head (Fig. 20A). Posterodorsal margin broadly rounded (Fig. 20A). Posterodorsal angle smooth, obtuse (Fig. 20A). Ventral margin of body convex, covered by setae of different size in different regions of valves (Figs. 20E–H, 22C). Anteroventral angle rounded. Valves with prominent sculpture, represented by polygons (Fig. 22D).
Head large (Fig. 20A–B), head length from tip of rostrum to border with valves makes up 0.30 times of body length. In lateral view, dorsal margin of head without dome above compound eye. Head ventral margin with projection or inflated (Fig. 20B). Compound eye significantly larger than ocellus (Fig. 20B). Dorsal head pore large, rounded (Figs. 20C, 22B). Labrum large, triangular in lateral view (Fig. 20D). Distal labral appendage finely setulated (Fig. 20D).
Thorax relatively long (Fig. 20A). Abdomen short (Fig. 20A).
Postabdomen elongated (Figs. 20I, 22E), subrectangular in lateral view; postabdomen length/height ratio about 3. Ventral margin of postabdomen almost straight, with transverse rows of fine setules (Figs. 20I–J, 22E). Preanal margin long, about in 3 times longer than anal margin. Postanal margin significantly shorter than anal margin (Figs. 20I–J, 22E). Preanal and anal margins covered by transverse rows of fine denticles (Figs. 20I–J, 22E). No prominent postabdominal flaps at side of anus (Fig. 20I–J). Postabdominal seta as long as postabdomen, its distal segment short, covered by long setules (Fig. 20I). Postabdominal claw small (almost subequal in length to postanal margin of postabdomen), curved, with pointed tip and broad base in lateral view (Figs. 20K, 22F). Several denticles on its dorsal side and more fine denticles on ventral side.
Antenna I rod-like (Figs. 21A, 24A) long and straight. Its inner margin covered by transverse rows of small denticles, also the whole surface of antennular body bears fine spinules (Figs. 21A, 24A). Antennular sensory seta slender, arising from outer side of proximal part (Fig. 21A). Nine aesthetascs, two of them longer and thicker than the rest. Each aesthetasc bears two minute “claws” at the apex (Fig. 21A).
Antenna II large (Figs. 21B, 22A, G–H), coxal region slightly folded, with two small sensory setae subequal in size (Fig. 21B). Antennal formula: setae 0-0-1-3/1-1-3, spines 0-1-0-1/0-0-1. Basal segment robust, conical, covered by transverse rows of fine spinules (Figs. 21B, 22A, G–H). Small spine (subequal in length to first exopod segment) located on outer surface of basal segment (Figs. 21B, 22G). Bisegmented short seta (subequal in length to first plus second exopod segments) located on inner surface of basal segment (Figs. 21B, 22H). Exopod and endopod branches subequal in size (Figs. 21B, 22A). All their segments cylindrical, elongated, covered by transverse rows of fine spinules (Fig. 22A). Apical swimming setae long subequal in length, bearing fine spinules and long setules (Figs. 21B, 22J–K). Lateral seta of proximal endopod segment (Fig. 21B–C) longer than other setae and armed with two rows of spinules: spinules on the edge of this seta are thin and densely located (distance between two neighboring spinules is almost equal to their length); spinules on the outer surface of this seta are more robust and sparsely located (distance between two neighboring spinules is in two times more than width of seta) (Figs. 21D–E, 22I). Seta on middle exopod segment reaches tips of apical setae, covered by long setules and fine stiff spinules (Fig. 21B). Lateral seta of third exopod segment has the same armature (Figs. 21B, 22K). True spine on second exopod segment thin, almost in three times shorter than third exopod segment (Figs. 21B, 22G–H). Second and third exopod segments bear short additional spines, and additional spines on third exopod segment are hardly visible under light microscope (Fig. 21B), but recognizable under scanning electron microscope (Fig. 22H). All investigated individuals had two-three additional spines on the second exopod segment and two significantly smaller additional spines on third exopod segment. Spines of both apical exopod and endopod segments thin, exopod apical spine in two times longer than endopod apical spine (Fig. 22K).
Thoracic limbs: five pairs (Fig. 23A–G).
Limb I large (Fig. 23A–B). Accessory seta short (Fig. 23B). ODL conical and large, bearing a single long bisegmented seta, its distal segment feathered unilaterally (Fig. 23B). IDL conical, covered by transverse rows of stiff setules, with three bisegmented setae of different size (Fig. 23B). Distal segment of each IDL seta covered unilaterally by stiff setules (Fig. 23B). Limb corm almost rectangular in lateral view (Fig. 23A). Endite 4 with three posterior soft setae (among them seta a the longest covered by long fine setules, setae b and c significantly shorter, subequal in size, bearing fine long setules in proximal parts and stiff short setules in distal parts) and a single stiff anterior seta 1 (Fig. 23A). Endite 3 with three soft posterior setae unequal in size and a single fork-like anterior seta 2 (Fig. 23A). Endite 2 with two posterior bisegmented setae subequal in length, covered by fine short setules, and a single anterior seta 3 represented by fork (Fig. 23A). Endite 1 with two soft setae. A single ejector hook with setulated distal segment (Fig. 23A).
Limb II triangular-rounded (Fig. 23C). Exopodite ovoid, covered by fine setules, and bearing a single long soft seta (Fig. 23C). Inner portion of limb II with eight scrapers, decreasing in size proximally (Fig. 23C). A deep incision between endite 2 and endite 1. Portion of gnathobase (= endite 1) bordering endite 2 somewhat inflated and bears a row of fine setules (Fig. 23C). Distal armature of gnathobase with four elements (Fig. 23C). Filter plate with four soft setae, subequal in length (Fig. 23C).
Limb III (Fig. 23D–E) with subrectangular exopodite, bearing a single lateral seta and three distal setae (among them, middle seta somewhat longer than others) (Fig. 23D). Distal endite with three anterior setae and small sensillae near seta 2 and seta 3 (Fig. 23E). Proximal endite with a small elongated sensillum and three setae subequal in length (Fig. 23E). Six setae on posterior face of limb (a–f) (among them seta a short and thick, covered by small spinules in its distal part, other five setae with fine long setules) (Fig. 23E). Distal armature of gnathobase with four elements (Fig. 23E). Filter plate absent (Fig. 23D).
Limb IV (Fig. 23F) with small rounded exopodite, bearing distally two soft setae, subequal in size. Inner distal portion with four anterior setae (1–4) and small sensillae near seta 2 and seta 3 (Fig. 23F). Posterior face with five soft setae increasing in size proximally (Fig. 23F). Distal armature of gnathobase consists of four elements (a small bottle-shaped sensillum, bisegmented seta and two small projections. Filter plate absent (Fig. 23F).
Limb V (Fig. 23G) with three-lobed densely setulated preepipodite. Epipodite ovoid. Exopodite with a single seta, covered by fine setules (Fig. 23G). Inner distal portion as a small flap, covered by setules; three setae on its inner margin (the distalmost seta significantly longer and thicker than others) (Fig. 23G). Filter plate absent (Fig. 23G).
Ephippial female. In lateral view, body proportions as in parthenogenetic female. Structure of ephippium typical for the genus (Fig. 24B–C). Almost all valves area incorporated to ephippium. Surface of ephippium with polygonal hillocks (Fig. 24B). Two eggs in ephippium.
Adult male. Body ovoid, dorsal margin interrupted by a shallow depression between head and valves (Fig. 25A). Dorsal margin of valves straight or slightly convex not elevated above head (Fig. 25A). Posterodorsal angle distinct, acute, without spine (Fig. 25A). Posteroventral region of valve rounded; ventral margin convex. Anteroventral angle broadly rounded (Fig. 25A). Head large, with small supraocular dome (Fig. 25B). Ventral margin of head slightly inflated (Fig. 25B).
Postabdomen subrectangular (Fig. 25C), with inflated ventral margin. Armature of dorsal margin similar to parthenogenetic female. Gonopores open on ventral sides near claws base (Figs. 24D, 25C).
Antenna I straight (Fig. 25D), rod-like, almost subequal in length to head. Antennular body covered by fine spinules and denticles of different length and thickness. Male seta located in the middle of antennular body on the inner side (Fig. 25D). Sensory seta located on the outer side of antennular body near its base (Fig. 25D). Apex of antenna I bears nine terminal aesthetascs, two of them significantly longer than others (Fig. 25D). Armature of proximal endopod segment seta of antenna II identical to that in parthenogenetic female (Fig. 25A).
Thoracic limb I (Fig. 25E) with ODL and IDL as in female (male seta was not found), copulatory hook elongated, curved, its distal portion with fringe.
Size. Maximum length of adult parthenogenetic females up to 1.00 mm, height 0.64 mm. Maximum length of ephippial females 0.77 mm, height 0.55 mm. Maximum length of adult males 0.46 mm, height 0.26 mm. Holotype is 0.83 mm in length, 0.55 mm in height. Allotype is 0.46 mm in length, 0.26 mm in height.
Variability. No significant variability was found between all investigated individuals.
Distribution. According to Smirnov and Timms (1983), regarding this taxon as M. capensis, M. australiensis sp. nov. is widely distributed in Australia, but most populations are located in the non-tropical portion of the continent: New South Walles, Victoria, South Australia and Tasmania.
Differential diagnosis. As M. australiensis sp. nov. is an endemic of Australia, here we analyze main differences of M. australiensis sp. nov. from other well-delineated Australian taxa, possible members of M. paulensis group. M. australiensis sp. nov. clearly differs from M. flagellata (Smirnov and Timms, 1983), M. schauinslandi Sars, 1904 and M. timmsi (Smirnov, 1976) in antenna II features (length of spine on the second exopod segment, number and length of additional spines, armature of the long seta on endopod proximal segment), as well as structure of postabdomen and postabdominal seta (see Tab. 2). According to some specific characteristic, M. australiensis sp. nov. seems to be closer to M. timmsi, than to other two species. M. australiensis sp. nov. and M. timmsi have postabdominal seta with short distal segment covered by long setules, while postabdominal seta of M. flagellata and M. schauinslandi bears long distal segment (subequal in length to proximal segment or even longer) (see Tab. 2). But M. australiensis sp. nov. differs from M. timmsi in structure of preanal margin of postabdomen. Preanal margin of M. timmsi is covered by very robust bifurcated denticles, while preanal margin of M. australiensis sp. nov. is covered by rows of small spinules (see Tab. 2). Main differences of M. australiensis sp. nov. from other well-delineated members of M. paulensis group are shown in Table 1 concerning mainly fine details (structure of antenna I and antenna II, thoracic limbs), as well as male morphology.
(3) Cladistic analysis
In the present analysis, 19 morphological characters in 12 taxa derived from our analysis of original samples and literature data were incorporated (Tabs. 3 and 4). Characters 6 and 7 were marked as ordered transformation series. Our analysis with M. triserialis as outgroup yielded 18 equally-parsimonious trees (TL = 31, CI = 0.807, RI = 0.842), a strict consensus tree is represented in Fig. 26. Bootstrap test resulted in a tree with the same topology.
All examined taxa form a monophyletic M. paulensis group with the following synapomorphies: 7, 10, 13, 16, 18. Within the latter, we can recognize: (1) a basal section (M. capensis, M. australiensis sp. nov., M. agsensis, M. atahualpa, M. smirnovi) with unclear relationships between each other (except M. atahualpa and M. smirnovi which are closest relatives); (2) a crown group with the following synapomorphies: 11, 15, 17. Within this group, two main clades are differentiated: (A) the Neotropical clade M. paulensis plus M. brandorffi, supported by the following synapomorphies: 4, 5, 6, 9, 10, and (B) a clade uniting Asian taxa plus Neotropical M. sioli, supported by the following synapomorphies: 6, 12, 19.
The homoplastic characters are: 1, 6, 8, 18.
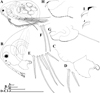 |
Fig. 1 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Adult parthenogenetic female, general view. B, Head. C, Labrum. D, Armature of anterior margin of valve. E, Armature of ventral margin of valve. F, Armature of posterior margin of valve. G, Postabdomen. H, Distal portion of postabdomen. I, Postabdominal claw, outer view. J, Postabdominal claw, inner view. Scale bars: 0.1 mm. |
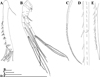 |
Fig. 2 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Antenna I. B, Antenna II. C, Lateral seta of basal endopod segment of antenna II. D, Its central part. E, Its distal part. Scale bars: 0.1 mm. |
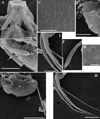 |
Fig. 3 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Head (ventral view). B, Dorsal pore. C, Central part of valve. D, Postabdomen. E, Distal portion of postabdomen. F–G, Antenna I. H, Antenna II. I, Exopod and endopod branches of antenna II. Scale bars: 0.2 mm for D, H, 0.1 mm for A, F–G, I, 0.05 mm for C, E, 0.01 mm for B. |
 |
Fig. 4 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Corm of limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. F, Limb IV. G, Limb V. Scale bars: 0.1 mm. |
 |
Fig. 5 Macrothrix capensis (Sars, 1916), females from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Parthenogenetic female. B–E, Ephippial female. A, Central part of lateral seta of basal endopod segment of antenna II. B, Ephippium (general view). C, Distal segments of antennal branches. D, Apical swimming setae. E, Fragment of ephippium. Scale bars: 0.2 mm for B, 0.1 mm for C, 0.02 mm for A, D–E. |
Comparison between Macrothrix paulensis-like species (based on original data and Sars, 1916; Harding, 1955; Idris and Fernando, 1981b; Ciros-Pérez and Elías-Gutiérrez, 1997; Dumont et al., 2002; Kotov and Hollwedel, 2004; Kotov et al., 2005, 2010; Garfias-Espejo et al., 2007).
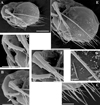 |
Fig. 6 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Lake Kud-Thing in floodplain of Mekong River, Nong Khai Province, Thailand, coll. 28.11.1998 by C. Saeng-aroon, AAK-2003-033. A, General view. B, Head. C, Armature of ventral margin of valve. D, Antenna I. E, Antenna II. F, Exopod and endopod branches of antenna II. G, Central part of lateral seta of basal endopod segment of antenna II. Scale bars: 0.2 mm for A, E, 0.1 mm for B, D, 0.05 mm for C, F, 0.2 mm for G. |
 |
Fig. 7 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, General view. B, Head. C, Labrum. D, Valve. E, Armature of anterior margin of valve. F, Armature of ventral margin of valve. G, Armature of posterior margin of valve. H, Postabdomen. I, Distal portion of postabdomen. J, Postabdominal seta. K, Postabdominal claw, outer view. L, Postabdominal claw, inner view. Scale bars: 0.1 mm. |
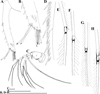 |
Fig. 8 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Antenna I. B, Distal portion of antenna I. C, Antenna II. D, Central part of lateral seta of basal endopod segment of antenna II. E, Central part of lateral seta of middle endopod segment of antenna II. F, Central part of lateral exopod segment seta. G–H, Apical swimming setae in different position. Scale bars: 0.1 mm. |
 |
Fig. 9 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A–B, Armature of ventral margin of valve. C, Central part of valve. D, Postabdomen. E–F, Distal portion of postabdomen. G, Postabdominal claws. H, Antenna I. Scale bars: 0.2 mm for D, 0.05 mm for A, E–F, H, 0.02 mm for B–C, G. |
 |
Fig. 10 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool, “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Antenna II. B, Exopod and endopod branches of antenna II. C–D, Proximal portion of lateral seta of basal endopod segment of antenna II. E–G, Central part of lateral seta of basal endopod segment of antenna II. Scale bars: 0.2 mm for A, 0.1 mm for B, 0.05 mm for E, 0.02 mm for C–D, F–G. |
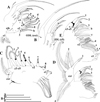 |
Fig. 11 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool, “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Corm of limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. Scale bars: 0.1 mm. |
 |
Fig. 12 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool, “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Limb IV. B, Fragment of limb IV. C, Limb V. Scale bars: 0.1 mm. |
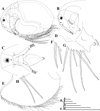 |
Fig. 13 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, General view. B, Head (lateral view). C, Head (ventral view). D, Labrum. E, Valve. F, Armature of anterior margin of valve. G, Armature of ventral margin of valve. H, Armature of posterior margin of valve. Scale bars: 0.1 mm. |
 |
Fig. 14 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, Postabdomen. B, Distal portion of postabdomen. C, Distal segment of postabdominal seta. D, Postabdominal claw, outer view. E, Postabdominal claw, inner view. F, Antenna I. G, Antenna II. H, Middle potion of lateral seta of basal endopod segment of antenna II. Scale bars: 0.1 mm. |
 |
Fig. 15 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, General view. B, Head. C, Armature of ventral margin of valve. D, Central part of valve. E, Postabdomen. F, Postabdominal claw. G, Antenna II. H–I, Central part of lateral seta of basal endopod segment of antenna II. J, Apical swimming setae of antenna II. K, Mandible. L, Limb I. Scale bars: 0.5 mm for A, 0.2 mm for B, G 0.1 mm for E, 0.05 mm for C–D, J, L, 0.02 mm for F, H–I, K. |
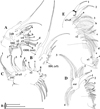 |
Fig. 16 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, Corm of limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. Scale bars: 0.1 mm. |
 |
Fig. 17 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, Limb IV. B, Fragment of limb IV. C, Limb V. Scale bars: 0.1 mm. |
 |
Fig. 18 Macrothrix odiosa Gurney, 1916 from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A–C, Ephippial female. D–E, Adult male. A, Ephippial female, general view. B, Ephippium, general view. C, Fragment of ephippium. D, Male, general view. E, Male, Head. Scale bars: 0.2 mm for A–B, D, 0.1 mm for E, 0.02 mm for C. |
 |
Fig. 19 Macrothrix odiosa Gurney, 1916, adult male from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, General view. B, Head. C, Postabdomen. D, Antenna I. E, Fragment of limb I. F, Male hook. Scale bars: 0.1 mm. |
 |
Fig. 20 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, General view. B, Head. C, Dorsal pore. D, Labrum. E, Valve. F, Armature of anterior margin of valve. G, Armature of ventral margin of valve. H, Armature of posterior margin of valve. I, Postabdomen. J, Distal portion of postabdomen. K, Postabdominal claw, outer view. Scale bars: 0.1 mm. |
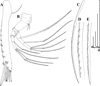 |
Fig. 21 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Antenna I. B, Antenna II. C, Lateral seta of basal endopod segment of antenna II. D, Its central part. E, Its distal part. Scale bars: 0.1 mm. |
 |
Fig. 22 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Valve. B, Dorsal pore. C, Armature of ventral margin of valve. D, Central part of valve. E, Postabdomen. F, Postabdominal claw. G–H, Exopod and endopod branches of antenna II. I, Central part of lateral seta of basal endopod segment of antenna II. J, Swimming setae of antenna II. K, Apical spines of antenna II. Scale bars: 0.5 mm for A, 0.1 mm for E, J–K, 0.05 mm for D, H, 0.02 mm for F, 0.01 mm for B–C, G, I. |
 |
Fig. 23 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. F, Limb IV. G, Limb V. Scale bars: 0.1 mm. |
 |
Fig. 24 Macrothrix australiensis sp. nov., from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Parthenogenetic female, ventral view (small spines on antenna I are marked via arrow). B–C, Ephippial female. D, Postabdomen of adult male (gonopores is marked via arrow). Scale bars: 0.1 mm for A, 0.2 mm for B–C, 0.02 for D. |
 |
Fig. 25 Macrothrix australiensis sp. nov., adult male from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, General view. B, Head. C, Postabdomen. D, Antenna I. E, Limb I. Scale bars: 0.1 mm. |
Comparison between Australian members of the genus Macrothrix Baird, 1843 (after Smith, 1909; Gurney, 1927; Smirnov and Timms, 1983; Smirnov, 1976 (we kept Russian letters for original illustrations); 1992 and our current data)
Character descriptions (p, present; a, absent).
Data matrix of 19-morphological characters in 12 taxa used in cladistic analysis. Data missing, or varying - .
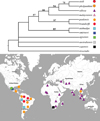 |
Fig. 26 A strict consensus of 18 equally-parsimonious trees for all investigated members of Macrothrix paulensis species group and map of their distribution (TL = 31, CI = 0.807, RI = 0.842). The 50% majority rule bootstrap simulation led a tree of similar topology with the contree. Due to this fact, branch probabilities were assigned to the aforementioned contree. |
4 Discussion
Diagnosing monophyletic Macrothrix paulensis species group
We offer several diagnostic characters of the M. paulensis species group among other members of the genus Macrothrix. First of all, these characters are important for the differentiation of the former from its possible congeners, the M. triserialis group (even some members of former were previously regarded as members of the latter, see Dumont et al., 2002).
Character 7 (see Tabs. 1, 3). A large, usually triangular labrum. Only M. capensis, M. agsensis and M. malaysiensis have a rounded labrum, other taxa of the M. paulensis group possess a triangular labrum. Usually all other taxa of Macrothrix have a small labrum (Smirnov, 1976, 1992; Kotov, 1999; Silva-Briano et al., 1999; Dumont et al., 2002; Kotov et al., 2004). This size and shape of labrum is widely used in the cladoceran taxonomy, see in Frey (1975, 1980), Smirnov (1996), Bekker et al. (2012).
Character 10 (see Tab. 3). Presence of robust denticles on the dorsal (concave) side of the postabdominal claw is a very characteristic of the M. paulensis group, moreover, two taxa – M. paulensis and M. brandorffi – have only robust denticles on the inner face of the postabdominal claw. Appearance of strong denticles on the postabdominal claws instead of fine setules is very characteristic for different daphniids: DaphniaO.F. Mueller (Alonso, 1996; Benzie, 2005), CeriodaphniaDana (Alonso, 1996), Scapholeberis Schoedler (Dumont and Pensaert, 1983). Two taxa, M. smirnovi and M. atahualpa, have a very specific armature of the postabdominal claw: a row of long and robust spines strongly increasing in size distally.
Character 13 (see Tab. 3). A single ejector hook on limb I in the M. paulensis group and two ejector hooks in the M. triserialis group. The latter character is especially important. All Anomopoda are characterized by two ejector hooks, but there are a few exceptions such as Ilyocryptus acutifrons group (Alonso, 1996; Kotov and Elías-Gutiérrez, 2009) and M. paulensis group in our new understanding, including M. atahualpa Brehm, 1936 (see a single ejector hook in Kotov et al., 2010: fig. 9d) and two taxa described above. Unfortunately, some previous descriptions of Macrothrix taxa (detailed in other traits!) (Ciros-Pérez and Elías-Gutiérrez, 1997; Dumont et al., 2002) are lacking an information on the ejector hook number.
Character 16 (see Tab. 3). Proximal endite of limb III with a small elongated sensillum and three setae in theM. paulensis group, but with a small bottle-shaped sensillum and four setae on proximal endite in the M. triserialis group. The lack of a seta of full length before three other setae of normal length may be considered as: (1) a complete reduction of the sensillum and partial reduction of seta 4; or (2) a sensillum behind seta 4 is kept, but seta 4 is completely reduced; (3) a sensillum and seta behind it are kept, and one of other setae completely reduced. We could not clarify this phenomenon without investigations of thoracic limbs development, but, interestingly, the same number of sensillae and setae on proximal endite of limb III is characteristic for other members of the M. paulensis group (Kotov and Hollwedel, 2004; Kotov et al., 2005).
Character 18 (see Tab. 3). Members of the M. paulensis group have no setae at the posterior surface of the thoracic limb IV gnathobase, members of the M. triserialis group bear an additional soft seta here. This seta is found in other Macrothrix species, having a dilated antenna I (see e.g. in Kotov, 2007b). But even basal members of the group, like M. atahualpa (Kotov et al., 2010), lacks this seta which makes these taxa similar to taxa of the crown group. Also M. atahualpa has five soft setae on inner-distal portion of limb IV (Kotov et al., 2010 noted four setae, but we refuted this opinion based on re-examination of the same samples).
Also, it is necessary to note that usually members of the group are large macrothricids (up to 1 mm). This character was not included to the cladistic analysis as difficult for an adequate analysis due to insufficient knowledge on the maximum size of the taxa under consideration.
Our cladistic analysis based both on original and previously published literature data led us to conclusion that M. capensis, M. atahualpa, M. smirnovi, M. agsensis and M. australiensis sp. nov. must be included to the M. paulensis group in our new understanding (Tab. 1). M. atahualpa obviously belongs to the M. paulensis species group as well. Take into consideration that the diagnostic features of M. atahualpa considered by Kotov et al. (2010) are not strong enough to oppose this species to other members of the M. paulensis group. We have already shown that the shape of ventral head margin varies within the group (it could be with a projection or without a projection, or somewhat inflated) (see Tabs. 1 and 2), the armature of the inner side of antenna I is also varying among taxa.
Main distinctive features of M. atahualpa and M. smirnovi as compared with other paulensis-like species are: (1) the specific armature of the postabdominal claw (see above) and (2) the presence of soft seta between scraper 4 and scraper 5 on limb II (we checked this feature in particular in some specimens) (see Tab. 1). In reality, the atahualpa-group needs to be revised once more, because M. smirnovi could be in fact a junior synonym of M. atahualpa. Descriptions of M. smirnovi by Ciros-Pérez and Elías-Gutiérrez (1997) and by Dumont et al. (2002) contradicts each other is some details, i.e. in male characters. Differences between M. smirnovi and M. atahualpa in our key (based on literature data on these taxa) could be illusory, appeared due to differences in the style of drawings of Harding (1955), Ciros-Pérez and Elías-Gutiérrez (1997) and Dumont et al. (2002).
Some characters of M. malaysiensis still remains unclear due to its incomplete description (Idris and Fernando, 1981b), but our cladistic search unambiguously attributed it to the paulensis-group.
Summarization of all available morphological data led us to the conclusion that morphological diversity of fine details within M. paulensis-like taxa is relatively strong, but these features are important for the species discrimination. The same situation, when fine details are critical for accurate and adequate identification, is known from many species groups, among which other large-bodied cladocerans like Eurycercus Baird or the Daphnia similis group (Bekker et al., 2012; Kotov and Bekker, 2016; Popova et al., 2016). In the future (after careful reexamination of all members of the genus Macrothrix) the taxa from the M. paulensis group could be attributed to the subgenus Iheringula within the genus Macrothrix (see also Kotov et al., 2005). However such a designation would depend on a wider revision of the (likely paraphyletic) Macrothrix, and not relevant in this study. In any case, investigators of Macrothrix species must be ready to dissect specimens for searching of fine details that are critical for accurate identification. Below we are giving a preliminary identification key for all known to date species of M. paulensis-group and some close taxa.
Key to known species of M. paulensis-group and some close taxa discussed above (modified after Kotov et al., 2005)
1 (2) Two contiguous spines near the base of antenna I − Macrothrix malaysiensis Idris and Fernando, 1981
(1) No spines on the base of antenna I – 3
3 (4) Posterodorsal angle of body smooth (or with small triangular spine), serration on the dorsum not expressed – 7
4 (3) Posterodorsal angle of body with a large triangular spine, serration on the dorsum is present − 5
5 (6) Postabdominal anal flaps are prominent, on exopodite of thoracic limb IV seta 2 is in three times longer than seta 1 – Macrothrix pholpunthini Kotov, Maiphae and Sanoamuang, 2005
6 (5) Postabdominal anal flaps absent, on exopodite of thoracic limb IV seta 2 only slightly longer than seta 1 – Macrothrix sioli (Smirnov, 1982)
7 (8) Antenna I with bunches of more or less fine denticles – 13
8 (7) Antenna I with a row of robust large denticles – 9
9 (10) Anal margin of postabdomen with bunches of fine denticles, without long hairs; the largest seta of antenna II with a row of robust denticles alternating with stiff setules – Macrothrix odiosa Gurney, 1916
10 (9) Anal margin of postabdomen with bunches of fine denticles and long hairs; the largest seta of antenna II with a row of robust denticles – 11
11 (12) Dorsal keel absent, postero-dorsal angle smooth, distal segment of postabdominal seta relatively long – Macrothrix paulensis (Sars, 1900)
12 (11) Dorsal keel well-developed, postero-dorsal angle as a spine, distal segment of postabdominal seta very short – Macrothrix brandorffi Kotov and Hollwedel, 2004
13 (14) Soft seta between scraper 4 and scraper 5 on thoracic limb II absent – 17
14 (13) Soft seta between scraper 4 and scraper 5 on thoracic limb II well-developed – 15
15 (16) Three terminal aesthetascs on male antenna I longer than others, sensory seta very large – Macrothrix atahualpa Brehm, 1936
16 (15) Two terminal aesthetascs on male antenna I longer than others, sensory seta relatively short – Macrothrix smirnovi Ciros-Pérez and Elías-Gutiérrez, 1997
17 (18) Middle portion of seta on proximal endopod segment armed by robuster spinules alternating with stiff setules – Macrothrix australiensis sp. nov.
18 (17) Middle portion of seta on proximal endopod segment armed by uniform spinules or spines – 19
19 (20) Middle portion of seta on proximal endopod segment armed by relatively strong, sparcely located spines – Macrothrix capensis (Sars, 1916)
20 (19) Middle portion of seta on proximal endopod segment armed by relatively small, dencely located spinules – Macrothrix agsensis Dumont, Silva-Briano and Subash Babu, 2002
Australia as a possible source of additional taxa of the M. paulensis group
Our knowledge on species diversity within the M. paulensis species group is probably incomplete. First of all, Australia and Tasmania could be a source of additional species from this group, as the number of Macrothrix species described from this region is really large. According to Kotov et al. (2013a), and taking in consideration our new data, thirteen species of the genus Macrothrix are currently accepted as valid from Australia and Tasmania (listed in chronological order) (also, see Tab. 2):
(1) M. spinosa King, 1853;
(2) M. schauinslandi Sars, 1904;
(6) M. hystrix Gurney, 1927;
(4) M. breviseta Smirnov, 1976;
(5) M. carinata (Smirnov, 1976);
(6) M. longiseta Smirnov, 1976;
(7) M. pectinata (Smirnov, 1976);
(8) M. timmsi (Smirnov, 1976);
(9) M. flagellata (Smirnov et Timms, 1983);
(10) M. williamsi (Smirnov et Timms, 1983);
(11) M. flabelligera Smirnov, 1992;
(12) M. indistincta Smirnov, 1992;
(13) M. australiensis sp. nov.
At least one more taxon, M. burstalis Smith, 1909, is considered as species inquirenda.
Morphology of aforementioned Australian taxa of Macrothrix was studied by previous authors (Smirnov and Timms, 1983; Smirnov, 1976, 1992) according to high standards for that time. But now is obvious that these descriptions lack some fine, but very significant details (i.e. in structure of antenna I, antenna II and thoracic limbs). Thus a re-examination of Australian Macrothrix is an important task for the macrothricid taxonomy. Here we conduct the only formal analysis of available data of other Australian taxa (Smirnov and Timms, 1983; Smirnov, 1976, 1992) in order to clarify a position of M. australiensis sp. nov. among them. We have no information on the number of ejector hooks on the thoracic limb I for most Australian species of Macrothrix. But most probably M. timmsi has a single ejector hook (see in Smirnov, 1976: fig. 117I). Just this feature is maximally important for separation of the M. paulensis group from close M. triserialis group and all other species with two ejector hooks. We know precisely that (1) normally members of M. paulensis group are relatively large cladocerans (up to 1 mm in length, although this character may be confusing: compare size for M. paulensis and M. sioli in Smirnov (1992) and in Kotov and Hollwedel (2004)) and (2) they have a rod-like (undilated) antenna I. Unfortunately, each of these two features does not allow us to separate any members of M. paulensis group from members of M. triserialis group, but taxa from the latter reach only 0.7 mm in length (Kotov et al., 2004), however a combination of both aforementioned characters could be helpful for revealing possible members of the M. paulensis group among incompletely described forms. We summarized data on Australian Macrothrix taxa in Tab. 2, paying special attention to their size and shape of antenna I, as well as to some other diagnostic features. Small-sized Macrothrix species with dilated antenna I apparently do not belong to the M. paulensis group, and we may exclude them from our comparison.
There are five relatively adequately described Australian species: M. breviseta, M. hystrix, M. indistincta, M. longiseta and M. spinosa. M. burstalis needs a reexamination, because its morphology is scarcely studied, but Smith's type material is probably lost. Most probably, Smith (1909) dealt with juveniles of another taxon. An individual illustrated in his figure has 0.4 mm in length and no eggs in the brood pouch, but due to a rod-like antenna I it may be considered as a taxon from the M. paulensis species group. Kotov and Hollwedel (2004) have already shown that M. mira (Smirnov, 1992) is a junior synonym of M. paulensis: Smirnov (1992) described juveniles of M. paulensis 0.39 mm in length as a separate taxon (see Kotov and Hollwedel, 2004).
Unfortunately, Smirnov (1976, 1992) measured only several individuals or only holotype of each taxon during his revision of the genus Macrothrix. It confuses now a species delimitation based on morphometry. Smirnov's style of measurements led to a situation when some species, potentially belonging to M. paulensis group, could have length less than 1 mm. But we expect that more taxa from Australia belong to the M. paulensis group. Presumably, three following Australian Macrothrix species: M. flagellata, M. schauinslandi and M. timmsi also belong to the M. paulensis species group, or are at least its closest relatives. Other species with rod-like antennas I needs a careful reexamination.
Therefore, more members of both M. paulensis and M. triserialis species groups could be hidden among Australian taxa. Probably, Australia is a center of diversity for both these groups.
5 Short notes on ecology
No detailed investigations on ecology of the M. paulensis group were performed to date. Ironically, although members of this group have a very peculiar appearance, they are exclusively rarely recorded in the hydrobiological and ecological studies. Probably, the reason is that applied ecological studies dealt only with few types of water bodies such as lakes, rivers, reservoirs and ponds. However, it seems that at least some members of the M. paulensis group are associated with paludal shallow fishless habitats (swamps, rice fields, temporary pools with developed vegetation belt) which are full of life mainly during wet season (Van Damme and Dumont, 2010; Van Damme and Sinev, 2013; our observations), or even with dystrophic water bodies. Van Damme and Dumont (2010) found M. paulensis in the water bodies of Lençóis Maranhenses (NE Brazil) under low pH (4.2) and very low oxygen content (0.95–0.99 mg/L O2). Sometimes members of the M. paulensis group may co-occur in the aforementioned types of habitats (i.e. M. paulensis and M. sioli in our material from Brazil and M. pholpunthini and M. odiosa in a sole sample from Thailand), and also co-occur with other small-bodied Macrothrix species (such as M. flabelligera, M. oviformis, M. spinosa, M. superaculeata, M. triserialis) as well as with some other littoral cladocerans (Alona sp., Chydorus sp., Ilyocryptus sp., Moinodaphnia sp., Pleuroxus sp., Simocephalus sp.). Paludal habitats may be considered as an ancient water body type for cladocerans (Kotov, 2013) and, undoubtedly, they deserve more attention among cladoceran experts.
6 Biogeography
The cladoceran-based zoogeography periodically (with large time gaps) attracts the attention of the hydrobiologists (e.g. Richard, 1892; Brehm, 1933; Frey, 1987; Chiambeng and Dumont, 2005; Korovchinsky, 2006; Van Damme and Sinev, 2013; Van Damme and Kotov, 2016). Development of the cladoceran taxonomy stimulates progress in biogeography and vice versa. Recently a new direction of such studies – phylogeography (or genogeography) (Avise, 2000; Hewitt, 2004) – became popular among the cladoceran investigators, but predominantly planktonic cladocerans are used as models for such analysis (e.g. Adamowicz et al., 2009; Faustová et al., 2011; Crease et al., 2012; Hamrová et al., 2012), while cladocerans with other mode of life attract insufficient attention (Belyaeva and Taylor, 2009; Kotov et al., 2016). In addition, mostly such studies are limited by the Holarctic region, with few exceptions (Sharma and Kotov, 2013).
It is necessary to take into consideration that the resting eggs/ephippia of the planktonic taxa are well accommodated to the dispersion, i.e. by water birds (see Kotov, 2013; Incagnone et al., 2014). Few attempts to explain biogeographical patterns for littoral cladocerans were conducted (e.g. Frey, 1987; Korovchinsky, 2006; Van Damme and Sinev, 2013; Kotov et al., 2016; Van Damme, 2016). But it is known that the resting stages of chydorids and macrothricids are not so strongly protected from the unfavorable influence of the environment and dispersed no so actively as the aforementioned resting stages of the planktonic cladocerans. As a result, biogeographical patterns in chydorids and macrothricids could be different from those previously revealed for the daphniids (Kotov, 2013; Van Damme and Sinev, 2013; Kotov et al., 2016).
The M. paulensis species group could be regarded as a model for biogeographical speculations based on morphological data due to: (1) a “pantropical” distribution (one of the intriguing distribution pattern in biogeography – see links in Van Damme and Sinev (2013)) and (2) a reasonable (not very small and not very large) number of species with well-recognizable morphological characteristics. Some important conclusions could be made from the analysis of biogeographical patterns in the M. paulensis-group:
(1) No truly “Pantropical” taxa were found within this group. Such conclusion was expected keeping in mind “Frey's non-cosmopolitanism” paradigm of recent cladoceran biogeography (see Frey, 1982, 1987). All the taxa from the M. paulensis-group could be classified as: (1) exclusively Neotropical (existence of very rare populations of M. paulensis in southernmost portion of North America can be easily explained by an expansion from South America); (2) exclusively Australian; (3) Palaeotropical (Afro-Asian); (4) endemics of Mexican plateau and closest territories (see Tab. 1, Fig. 26). As we told above, more members of the paulensis-group could be present in Australia, but their revealing will not change the aforedescribed pattern. Such patterns were found in other cladoceran taxa previously regarded as “cosmopolitan” (Dumont and Silva-Briano, 2000; Sinev et al., 2005; Van Damme et al., 2011; Sharma and Kotov, 2013; Neretina and Sinev, 2016; Neretina and Kotov, 2017).
(2) Five members of the basal section of the tree (M. australiensis sp. nov., M. atahualpa, M. smirnovi, M. capensis and M. agsensis) are endemics of four well-recognised centres of endemism of the Cladocera: Australian centre (see Smirnov and Timms, 1984; Hebert and Wilson, 1994; Korovchinsky, 2006; Forró et al., 2008), Andean highland centre (Kotov et al., 2010), Mexican plateau centre (Elías-Gutiérrez et al., 2001; Kotov et al., 2003; Korovchinsky, 2006; Garfias-Espejo et al., 2007) and South African centre (see Van Damme et al., 2013b) (Tab. 1, Fig. 26). If the structure of the former two centres of endemism is poorly known, the last centre is relatively well-studied. M. capensis belongs to the third group of the South African endemics by Van Damme et al. (2013b): taxa widely distributed in the fourth, both in the mountains and in the lowlands. See further comments on this zone of endemism in Van Damme et al. (2013b). It is important, that all five aforementioned endemic taxa (M. australiensis sp. nov., M. atahualpa, M. smirnovi, M. capensis and M. agsensis) are obvious both phylogenetic and biogeographical relicts (Purvis et al., 2005). They could be regarded as “ejected relicts” sensu Korovchinsky (2006) as their distribution ranges correspond well to this model. These taxa are palaeoendemics sensu Harrison (1965).
(3) The crown group, in contrast, is distributed in the lowlands of South America, Africa and Asia with the range overlapping with members of the basal section only in South Africa (Tab. 1, Fig. 26). The crown group is subdivided into two sub-groups: exclusively Neotropical clade and a predominantly Afro-Asian clade which also includes a single Neotropical taxon. Such pattern is apparently old (see Van Damme et al. (2013b)). It could be associated with events like Gondwana break up, or even with older times of Pangaea break up followed by a subsequent extinction in the northern hemisphere.
Korovchinsky (2006) argued that a chance to trace some “Gondwanian” events is too small due to subsequent mid-late Caenozoic extinctions, and we agree with this idea. Indeed, a chance to distinguish between different scenarios is extremely low keeping in mind that some arguments pro and contra such scenarios could be obtained only from some fossil records. But to date such records are rare, although it could be partly explained by an insufficient attention of carcinologists to fossil collections (Kotov and Korovchinsky, 2006; Kotov, 2007a; Van Damme and Kotov, 2016). The molecular clocks sometimes are regarded as a panacea for dating and discerning between different scenarios, but such approach is strongly vulnerable for a criticism as is needed in a very accurate calibration based on fossil records (Heads, 2005; Pulquerio and Nichols, 2007) keeping in mind very strong differences in the mutation rates of different genes in different groups of organisms and un-regularities (Ho et al., 2005, 2015). Therefore a really accurate molecular clock could be proposed only in cases of future fossil records (Van Damme and Kotov, 2016). Again, an un-proportional extinction on different continents could be regarded as an alternative version to any vicariant scenarios, but it is well-known the latter approach could explain all possible scenarios (see “transatlantic domino” offered by Eskov (1984)).
(4) Within the crown group, there are two main sub-groups, and the version on the inter-continental differentiation of each group, correspondingly in South America and in Asia (= inhabited by of a common ancestor for each group on a separate continent), seems to be the most parsimonious (Tab. 1, Fig. 26). The groups, among other territories, occupy two main centres of the cladoceran diversity in tropics: Brazilian tropical lowlands and South-East Asian lowlands. These regions are intensively studied recently, specially for the chydorids (Kotov et al., 2004, 2005; Sinev et al., 2004, 2005; Sinev and Sanoamuang, 2007; Van Damme et al., 2011; Sinev and Kotov, 2012; Van Damme and Maiphae, 2013; Van Damme and Sinev, 2013; Van Damme et al., 2013a; Dumont et al., 2013; Elmoor-Loureiro, 2014; Sinev et al., 2016; Sousa et al., 2015, Sousa et al., 2016a,b). But studies of the biodiversity structure in the Brazilian tropical lowlands and SE Asian lowlands need to be continued. To date we cannot speak about presence/absence of an analogous biodiversity center in African tropical lowlands due to a clearly insufficient study of this region. Moreover, previous ideas on the lower diversity of the cladocerans in African tropical zone (Dumont, 1994; Chiambeng and Dumont, 2005) could be an artifact of an insufficient level of study of the African cladoceran fauna (Van Damme and Dumont, 2009).
(5) It is necessary to take into consideration that “the existence of antique lineages does not contradict with the possibility of recent speciation and adaptation” (Van Damme et al., 2013b). To date we cannot say, are endemic taxa from the regions marked above (in item 4) relicts, or products of a secondary intensive speciation in these territories? The second version seems to be most likely for us: locally distributed endemics in both sub-groups (M. sioli, M. pholpunthini and M. malaysiensis) are biogeographical, but not phylogenetic relicts (as they are relatively “advanced” taxa in our tree, the members of the crown group) (Tab. 1, Fig. 26).
(6) A close relationship between M. pholpunthini and M. sioli (Tab. 1, Fig. 26) could be explained by a secondary expansion from Asia to South America through the “boreotropical migration hypothesis”, see expanded notes on this subject in Van Damme and Sinev (2013).
Among possible scenarios explaining recent distribution of the taxa of the paulensis-group, some Mesozoic scenarios are preferable according to several reasons (see above). Our main conclusion from the analysis of the biogeographical patterns in the M. paulensis group is that all these scenarios are very old, they are related to some late Mesozoic – mid Caenozoic tectonic events, as it was also proposed for some other cladocerans (Frey, 1982, 1987; Popova et al., 2016). It means that the genus Macrothrix is of at least a Mesozoic origin, similarly to other taxa of the Anomopoda and Ctenopoda (Sacherová and Hebert, 2003; Kotov and Korovchinsky, 2006; Kotov and Taylor, 2011; Van Damme and Kotov, 2016). To our opinion, a combination of different scenarios took place in the evolutionary history of M. paulensis group. A single approach as a “mobilistic biogeography” or “ejected relict” version could not give a realistic explanation of all diversity of the biogeographical patterns in the Cladocera.
Remarkably, similar patterns were alreary revealed in some other cladocerans like chydorid Anthalona Van Damme, Sinev and Dumont (Van Damme et al., 2011) or ilyocryptid Ilyocryptus Sars (Kotov and Elías-Gutiérrez, 2009). Obviously, studies of other cladoceran genera are necessary to confirm a universallity of such pattern among the taxa previously regard as pan-tropical ones.
Macrothrix is the most diverse genus of the Macrothricidae. But the macrothricid-based zoogeographical reconstructions may not be limited only by this genus Macrothrix, see Frey (1988), other genera could also be a subject of a biogeographical analysis (Neretina and Kotov, 2017). It is necessary to take into consideration that ideas on the macrothricids as a “primitive” group (Behning, 1941) are quite superficial. They (after separation of Acantholeberidae, Ophryoxidae and Ilyocryptidae) form a specific portion of the crown-group of the suborder Radopoda (Dumont and Silva-Briano, 1998; Kotov, 2013).
Distribution patterns on the genus level could be interesting as well (Korovchinsky, 2004). It is already obvious that some macrothricids and macrothricid-like genera are associated with particular zoogeographical regions. For instance, macrothricid-like anomopods Acantholeberis Lilljeborg, Ophryoxus Sars, Parophryoxus Doolitle and macrothricids Bunops Birge, Drepanothrix Sars, are typical for the Holarctic region (Forró et al., 2008). Neothrix Gurney is considered to be an endemic of the Australian region; the rare Cactus Smirnov is an endemic of Tierra del Fuego. But initial opinion on the continental endemicity of some other macrothricid genera (Smirnov, 1976) was false. Onchobunops Fryer and Paggi was considered as an endemic of the Neotropics, but it was subsequently found in SE Asia (Tanaka and Ohtaka, 2010). The genus Lathonura Lilljeborg was regarded as Holarctic, but it was subsequently found in South Africa (Hart and Dumont, 2005), although this case could be explained by a human-mediated invasion. Therefore, the investigations of the macrothricids are potentially interesting for analysis of cladoceran distribution patterns, but such studies need be continued using morphological and genetic methods.
Acknowledgements
We are much indebted to Prof. Nikolai N. Smirnov for help in the investigations of the M. paulensis group; to Prof. Geoff Boxshall for information on absence of any G.W. Smith's samples in the collection of NHM, Dr. Kay Van Damme and anonymous reviewer for valuable comments on earlier draft; to Mr. Wondie Zelalem for samples with M. odiosa. Many thanks to Mr. Sergei I. Metelev and Mr. Alexey N. Nekrasov for their assistance to ANN during SEM works. SEM works were carried out at the Joint Usage Center “Instrumental methods in ecology” at A.N. Severtsov Institute of Ecology and Evolution. ANN was supported by award from President of Russian Federation on 2016/2017 academic year (order no 1184 from 12.09.2016). AAK was supported by Russian Government Program of Competitive Growth of Kazan Federal University.
References
- Adamowicz SJ, Petrusek A, Colbourne JK, Hebert PDN, Witt JDS. 2009. The scale of divergence: a phylogenetic appraisal of intercontinental allopatric speciation in a passively dispersed freshwater zooplankton genus. Mol Phyl Evol 50: 423–436. [CrossRef] [Google Scholar]
- Alonso M. 1996. Crustacea, Branchiopoda. Fauna Iberica 7. Crustacea Branchiopoda. Museo Nacional de Ciencias Naturales. Madrid: Consejo Superior de Investigaciones Científicas, 486 p. [Google Scholar]
- Avise JC. 2000. Phylogeography: the history and formation of species. Cambridge: Harvard University Press, 464 p. [Google Scholar]
- Baird W. 1850. The natural history of the British Entomostraca. London: The Ray Society, 364 p. [Google Scholar]
- Behning AL. 1938. Elements of subtropical fauna into rice fields of Uzbekistan. Doklady AN SSSR 21: 293–296 [in Russian]. [Google Scholar]
- Behning AL. 1941. The Cladocerans of the Caucasus. Tbilisi: Gruzmedgiz Publ, 384 p. [in Russian]. [Google Scholar]
- Bekker EI, Kotov AA, Taylor DJ. 2012. A revision of the subgenus Eurycercus (Eurycercus) Baird, 1843 emend. nov. (Cladocera: Eurycercidae) in the Holarctic with the description of a new species from Alaska. Zootaxa 3206: 1–40. [Google Scholar]
- Belyaeva M, Taylor DJ. 2009. Cryptic species within the Chydorus sphaericus species complex (Crustacea: Cladocera) revealed by molecular markers and sexual stage morphology. Mol Phyl Evol 50: 534–546. [Google Scholar]
- Benzie JAH. 2005. The genus Daphnia (including Daphniopsis) (Anomopoda: Daphniidae). Guides to the identification of the microinvertebrates of the continental waters of the world 21. Leiden: Kenobi Productions, Ghent and Backhuys Publishers, 376 p. [Google Scholar]
- Biswas S. 1971. Fauna of Rajasthan, India. Part II. Crustacea: Cladocera. Rec Zool Surv India 63: 95–140. [Google Scholar]
- Brehm V. 1930. Notizen zur Cladocerenfauna Madagaskars. Arch Hydrobiol 21: 679–686. [Google Scholar]
- Brehm V. 1933. Die Cladoceren der Deutschen Limnologischen Sunda-Expedition. Arch Hydrobiol Suppl 11: 631–771. [Google Scholar]
- Brehm V. 1934. II. Cladoceren. Voyage de Ch. Alluaud & P. A. Chappius en Afrique Française. Arch Hydrobiol 26: 50–90. [Google Scholar]
- Brehm V. 1952. Cladoceren und calanoide Kopepoden von Madagascar. Mém Inst Sci Madagascar Ser A 7: 37–46. [Google Scholar]
- Cervantes-Martínez A, Gutiérrez-Aguirre M, Elías-Gutiérrez M. 2000. Description of Ilyocryptus nevadensis (Branchiopoda, Anomopoda), a new species from a high altitude crater lake in the volcano Nevado de Toluca, Mexico. Crustaceana 73: 311–321. [CrossRef] [Google Scholar]
- Chiambeng GY, Dumont HJ. 2005. The Branchiopoda (Crustacea: Anomopoda, Ctenopoda and Cyclestherida) of the rain forests of Cameroon, West Africa: low abundances, few endemics and a boreal-tropical disjunction. J Biogeogr 32: 1611–1620. [CrossRef] [Google Scholar]
- Ciros-Pérez J, Elías-Gutiérrez M. 1997. Macrothrix smirnovi, a new species (Crustacea: Anomopoda: Macrothricidae) from Mexico, a member of the M. triserialis group. Proc Biol Soc Wash 110: 115–127. [Google Scholar]
- Ciros-Pérez J, Silva-Briano M, Elías-Gutiérrez M. 1996. A new species of Macrothrix (Anomopoda: Macrothricidae) from Central Mexico. Hydrobiologia 319: 159–166. [CrossRef] [Google Scholar]
- Crease TJ, Omilian AR, Costanzo KS, Taylor DJ. 2012. Transcontinental phylogeography of the Daphnia pulex species complex. PLoS ONE 7: e46620. [CrossRef] [PubMed] [Google Scholar]
- Dumont HJ. 1994. On the diversity of the Cladocera in the tropics. Hydrobiologia: 272, 27–38. [CrossRef] [Google Scholar]
- Dumont HJ, Pensaert J. 1983. A revision of the Scapholeberinae (Crustacea: Cladocera). Hydrobiologia 100: 3–45. [CrossRef] [Google Scholar]
- Dumont HJ, Silva-Briano M. 1998. A reclassification of the anomopod families Macrothricidae and Chydoridae, with the creation of a new suborder, the Radopoda (Crustacea: Branchiopoda). Hydrobiologia 384: 119–149. [CrossRef] [Google Scholar]
- Dumont HJ, Silva-Briano M. 2000. Karualona n.gen. (Anomopoda: Chydoridae), with a description of two new species, and a key to all known species. Hydrobiologia 435: 61–82. [CrossRef] [Google Scholar]
- Dumont HJ, Van de Velde I. 1977. Cladocères et Conchostracéc гécoltes par le professeur Th. Monod dans la moyenne vallée du Niger en décembre 1972 et janvier 1973. Bull Inst Fondam Afr Noire (sér. A) 39: 75–93. [Google Scholar]
- Dumont HJ, Silva-Briano M, Babu KKS. 2002. A re-evaluation of the Macrothrix rosea-triserialis group, with the description of two new species (Crustacea Anomopoda: Macrothricidae). Hydrobiologia 467: 1–44. [CrossRef] [Google Scholar]
- Dumont HJ, Rietzler AC, Kalapothakis E. 2013. Micromoina arboricola n. gen., n. spec. (Crustacea: Cladocera), a new moinid living in a forest tree-hole in Minas Gerais, Brazil. Zootaxa 3652: 533–546. [CrossRef] [PubMed] [Google Scholar]
- Elías-Gutiérrez M, Smirnov NN. 2000. Macrothrix marthae, a new species (Crustacea: Anomopoda: Macrothricidae), a highly specialized macrothricid from Mexico. Proc Biol Soc Wash 113: 652–660. [Google Scholar]
- Elías-Gutiérrez M, Valdez-Moreno M. 2008. A new cryptic species of Leberis Smirnov, 1989 (Crustacea, Cladocera, Chydoridae) from the Mexican semi-desert region, highlighted by DNA barcoding. Hidrobiológica 18: 63–74. [Google Scholar]
- Elías-Gutiérrez M, Suárez-Morales E, Sarma SSS. 2001. Diversity of the freshwater zooplankton in the neotropics: the case of Mexico. Verh Int Verein Limnol 27: 4027–4031. [Google Scholar]
- Elías-Gutiérrez M, Jerónimo FM, Ivanova NV, Valdez-Moreno M, Hebert PDN. 2008. DNA barcodes for Cladocera and Copepoda from Mexico and Guatemala, highlights and new discoveries. Zootaxa 1839: 1–42. [Google Scholar]
- Elmoor-Loureiro LMA. 2014. Ephemeroporus quasimodo sp. nov. (Crustacea: Cladocera: Chydoridae), a new species from the Brazilian Cerrado. Zootaxa 3821: 88–100. [CrossRef] [PubMed] [Google Scholar]
- Eskov KY. 1984. Continental drift and problems of historical biogeography. In: Chernov YI, ed. Faunogenez i filocenogenez. Moskva: Nauka, pp. 24–92 [in Russian]. [Google Scholar]
- Faustová M, Sacherová V, Svensson J-E, Taylor DJ. 2011. Radiation of European Eubosmina (Cladocera) from Bosmina (E.) longispina − concordance of multipopulation molecular data with paleolimnology. Limnol Oceanogr 56: 440–450. [CrossRef] [Google Scholar]
- Fernando CH. 1980. The freshwater zooplankton of Sri Lanka, with a discussion of tropical freshwater zooplankton composition. Int Rev Ges Hydrobiol 65: 85–125. [CrossRef] [Google Scholar]
- Fernando CH, Kanduru A. 1984. Some remarks on the latitudinal distribution of Cladocera on the Indian subcontinent. Hydrobiologia 113: 69–76. [Google Scholar]
- Forró L, Korovchinsky NM, Kotov AA, Petrusek A. 2008. Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595: 177–184. [CrossRef] [Google Scholar]
- Frey DG. 1975. Subgeneric differentiation within Eurycercus (Cladocera, Chydoridae) and a new species from Northern Sweden. Hydrobiologia 46: 263–300. [CrossRef] [Google Scholar]
- Frey DG. 1980. On the plurality of Chydorus sphaericus (O.F. Müller) (Cladocera, Chydoridae) and designation of a neotype from Sjaelsø, Denmark. Hydrobiologia 69: 83–123. [CrossRef] [Google Scholar]
- Frey DG. 1982. Questions concerning cosmopolitanism in Cladocera. Arch Hydrobiol 93: 484–502. [Google Scholar]
- Frey DG. 1987. The taxonomy and biogeography of the Cladocera. Hydrobiologia145: 5–17. [CrossRef] [Google Scholar]
- Frey DG. 1988. Are there tropicopolitan macrothricid Cladocera? Acta Limnol Brasil 2: 513–525. [Google Scholar]
- Garfias-Espejo T, Elias-Gutierrez M, Silva-Briano M. 2007. On Macrothrix agsensis Dumont, Silva-Briano & Babu, 2002 (Cladocera: Anomopoda: Macrothricidae), with description of the male and ephippial females, and comments on the distribution of the genus in Mexico. Zootaxa 1632: 49–60. [Google Scholar]
- Gauthier H. 1930. Mission Saharienne Augiéras-Draper, 1927–1928. Cladocères, Ostracodes, Phyllopodes, Anostracés et Conchostracés. Bull Mus D'Hist Natur Paris 2: 92–99. [Google Scholar]
- Gurney R. 1907. Further notes on Indian freshwater Entomostraca. Rec Indian Mus 1: 21–33. [Google Scholar]
- Gurney R. 1916. On some fresh-water Entomostraca from Ceylon. Proc Gen Meet Sci Busin Zool Soc 1: 333–343. [Google Scholar]
- Gurney R. 1927. Some Australian freshwater Entomostraca reared from dried mud. Proc Gen Meet Sci Busin Zool Soc 1: 59–79. [Google Scholar]
- Hamrová E, Krajicek M, Karanovic T, Černý M, Petrusek A. 2012. Congruent patterns of lineage diversity in two species complexes of planktonic crustaceans, Daphnia longispina (Cladocera) and Eucyclops serrulatus (Copepoda), in East European mountain lakes. Zool J Linn Soc 166: 754–767. [CrossRef] [Google Scholar]
- Harding JP. 1955. Percy Sladen Trust expedition. XIX. Crustacea: Cladocera. Trans Linn Soc Lond 3: 329–354. [Google Scholar]
- Harrison AD. 1965. Geographical distribution of riverine invertebrates in Southern Africa. Arch Hydrobiol 61: 387–394. [Google Scholar]
- Hart RC, Dumont HJF. 2005. An Holarctic taxon in the Ethiopian region – a first record of Lathonura (Crustacea: Cladocera: Macrothricidae) of the Okavango swamps of subtropical Africa. S Afr J Sci 101: 565–567. [Google Scholar]
- Heads M. 2005. Dating nodes on molecular phylogenies: a critique of molecular biogeography. Cladistics 21: 62–78. [CrossRef] [Google Scholar]
- Hebert PDN, Wilson CC. 1994. Provincialism in plankton: endemism and allopatric speciation in Australian Daphnia. Evolution 48: 1333–1349. [CrossRef] [PubMed] [Google Scholar]
- Hewitt GM. 2004. The structure of biodiversity – insights from molecular phylogeography. Front Zool 1: 1–16. [CrossRef] [PubMed] [Google Scholar]
- Ho SY, Phillips MJ, Cooper A, Drummond AJ. 2005. Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol Biol Evol 22: 1561–1568. [CrossRef] [PubMed] [Google Scholar]
- Ho SY, Tong KJ, Foster CS, Ritchie AM, Lo N, Crisp MD. 2015. Biogeographic calibrations for the molecular clock. Biol Lett 11: 20150194. [CrossRef] [PubMed] [Google Scholar]
- Hudec I. 1993. Redescription of Daphnia deserti (Gauthier, 1937) (Crustacea: Daphniiformes: Daphniidae). Hydrobiologia 264: 153–158. [CrossRef] [Google Scholar]
- Ibrasheva SI, Smirnova VA. 1983. Kladotsera Kazahstana. Alma-Ata: Mektep Publishing, 136 p. [Cladocerans of Kazakhstan: in Russian]. [Google Scholar]
- Idris BAG. 1983. Freshwater zooplankton of Malaysia (Crustacea: Cladocera). Pertanian, Malaysia: Perenbit University, 153 p. [Google Scholar]
- Idris BAG, Fernando CH. 1981a. Cladocera of Malaysia and Singapore with new records, redescriptions and remarks on some species. Hydrobiologia 77: 233–256. [CrossRef] [Google Scholar]
- Idris BAG, Fernando CH. 1981b. Two new species of cladoceran crustaceans of the genera Macrothrix Baird and Alona Baird from Malaysia. Hydrobiologia 76: 81–85. [CrossRef] [Google Scholar]
- Incagnone G, Marrone F, Barone R, Robba L, Naselli-Flores L. 2014. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750: 103–123. [CrossRef] [Google Scholar]
- Korovchinsky NM. 1996. How many species of Cladocera are there? Hydrobiologia 321: 191–204. [CrossRef] [Google Scholar]
- Korovchinsky NM. 2004. Cladocerans of the order Ctenopoda of the world fauna (morphology, systematics, ecology, biogeography). Moscow: KMK Press, 410 p. [in Russian]. [Google Scholar]
- Korovchinsky NM. 2006. The Cladocera (Crustacea: Branchiopoda) as a relict group. Zool J Linn Soc 147: 109–124. [CrossRef] [Google Scholar]
- Kořínek V. 1984. Cladocera. Hydrobiological Survey of Lake Bangweulu and Luapulu River Basin. J Symoens Cercle Hydrobiol Brux 13: 1–117. [Google Scholar]
- Kotov AA. 1999. Redescription of Macrothrix tripectinata Weisig, 1934 (Anomopoda, Branchiopoda), with a discussion of some features rarely used in the systematics of the genus. Hydrobiologia 403: 63–80. [CrossRef] [Google Scholar]
- Kotov AA. 2007a. Jurassic Cladocera (Crustacea, Branchiopoda) with a description of an extinct Mesozoic order. J Nat Hist41: 13–37. [CrossRef] [Google Scholar]
- Kotov AA. 2007b. Revision of the hirsuticornis-like species of Macrothrix Baird, 1843 (Cladocera: Anomopoda: Macrothricidae) from Subantarctic and temperate regions of the southern hemisphere. J Nat Hist 41: 2569–2620. [CrossRef] [Google Scholar]
- Kotov AA. 2008. Importance of male and ephippial female characters for differentiating three Palaearctic species of Macrothrix Baird, 1843 (Cladocera: Anomopoda), with a redescription of Macrothrix dadayi Behning, 1941. Ann Limnol 44: 45–61. [Google Scholar]
- Kotov AA. 2013. Morphology and phylogeny of Anomopoda (Crustacea: Cladocera). Moscow: KMK, 638 p. [in Russian with English abstract]. [Google Scholar]
- Kotov AA, Bekker EI. 2016. Cladocera: family Eurycercidae (Branchiopoda: Cladocera: Anomopoda). In: Dumont HJ, ed. Identification guides to the plankton and benthos of inland waters, Vol. 25. Weikersheim: Backhuys Publishers, Leiden & Margraf Publishers, 89 p. [Google Scholar]
- Kotov AA, Elías-Gutiérrez M. 2009. A phylogenetic analysis of Ilyocryptus Sars, 1862 (Cladocera: Ilyocryptidae). Int Rev Hydrobiol 94: 208–225. [CrossRef] [Google Scholar]
- Kotov AA, Hollwedel W. 2004. Revision of the Macrothrix paulensis species group (Anomopoda, Cladocera, Branchiopoda) in Neotropics, with description of M. brandorffi n. sp. Arch Hydrobiol Suppl 151: 125–159. [Google Scholar]
- Kotov AA, Korovchinsky NM. 2006. First record of fossil Mesozoic Ctenopoda (Crustacea, Cladocera). Zool J Linn Soc 146: 269–274. [CrossRef] [Google Scholar]
- Kotov AA, Taylor DJ. 2010. A new African lineage of the Daphnia obtusa group (Cladocera: Daphniidae) disrupts continental vicariance patterns. J Plankt Res 32: 937–949. [CrossRef] [Google Scholar]
- Kotov AA, Taylor DJ. 2011. Mesozoic fossils (>145 Mya) suggest the antiquity of the subgenera of Daphnia and their coevolution with chaoborid predators. BMC Evol Biol 11: 129. [CrossRef] [PubMed] [Google Scholar]
- Kotov AA, Elías-Gutiérrez M, Nieto MG. 2003. Leydigia louisi louisi Jenkin, 1934 in the Neotropics, L. louisi mexicana n. subsp. in the Central Mexican highlands. Hydrobiologia 510: 239–255. [CrossRef] [Google Scholar]
- Kotov AA, Garfias-Espejo T, Elías-Gutiérrez M. 2004. Separation of two Neotropical species: Macrothrix superaculeata (Smirnov, 1982) versus M. elegans Sars, 1901 (Macrothricidae, Anomopoda, Cladocera). Hydrobiologia 517: 61–88. [CrossRef] [Google Scholar]
- Kotov AA, Maiphae S, Sanoamuang L. 2005. Revision of Macrothrix paulensis-like species (Anomopoda, Cladocera, Branchiopoda) in Asia, and phylogeny of the paulensis-group. Arch Hydrobiol 151: 269–299. [Google Scholar]
- Kotov AA, Sinev AY, Berrios VL. 2010. The Cladocera (Crustacea: Branchiopoda) of six high altitude water bodies in the North Chilean Andes, with discussion of Andean endemism. Zootaxa 2430: 1–66. [Google Scholar]
- Kotov AA, Forró L, Korovchinsky NM, Petrusek A. 2013a. World checklist of freshwater Cladocera species. World Wide Web electronic publication. Available from: http://fada.biodiversity.be/group/show/17 [last consulted on 2017/08/01] [Google Scholar]
- Kotov AA, Van Damme K, Bekker EI, et al. 2013b. Cladocera (Crustacea: Branchiopoda) of Vientiane province and municipality, Laos. J Limnol 72: 81–108. [Google Scholar]
- Kotov AA, Karabanov DP, Bekker EI, Neterina TV, Taylor DJ. 2016. Phylogeography of the Chydorus sphaericus group (Cladocera: Chydoridae) in the Northern Palearctic. PLoS ONE 11: e0168711. [Google Scholar]
- Kurz W. 1875. Dodekas neue Cladoceren nebst kurzen Übersicht der Cladocerenfauna Böhmens. Sitz Math-Naturwiss Cl Akad Wiss 70: 7–88. [Google Scholar]
- Laforsch C, Tollrian R. 2000. A new preparation technique of daphnids for Scanning Electron Microscopy using hexamethyldisilazane. Arch Hydrobiol 149: 587–596. [CrossRef] [Google Scholar]
- Löffler H. 1968. Die Crustaceenfauna der Binnengewässer ostafrikanischen Hochberge. Hochgebirgsforschung Heft 1: 107–170. [Google Scholar]
- Manujlova EF. 1964. Vetvistoysie rachki fauni SSSR [The cladocerans of fauna of the USSR]. Opredeliteli po faune SSSR 88: 1–327 [in Russian]. [Google Scholar]
- Meschiatti AJ, Arcifa MS. 2002. Early life stages of fish and the relationships with zooplankton in a tropical Brazilian reservoir: Lake Monte Alegre. Braz J Biol 62: 41–50. [CrossRef] [Google Scholar]
- Michael RG, Sharma BK. 1988. Indian Cladocera. (Crustacea: Branchiopoda Cladocera). Fauna of India and adjacent countries. The Technical and General Press, Calcutta, India, 262 p. [Google Scholar]
- Mukhamediev AM. 1986. Rakoobraznije vodoyernov Ferganskoy doliny [Crustaceans of the waters of Fergana valley]. Tashkent: FAN Press, 160 p. [in Russian] [Google Scholar]
- Neretina AN, Kotov AA. 2015. A new species of Acroperus Baird, 1843 (Cladocera: Chydoridae) from Africa. Zootaxa 4039: 516–528. [CrossRef] [PubMed] [Google Scholar]
- Neretina AN, Kotov AA. 2017. Old World-New World differentiation of so-called “circumtropical” taxa: the case of Grimaldina Richard, 1892 (Branchiopoda: Cladocera: Macrothricidae). Zootaxa 4291: 295–323. [CrossRef] [Google Scholar]
- Neretina AN, Sinev AY. 2016. A revision of the genus Leberis Smirnov, 1989 (Cladocera: Chydoridae) in the Old World and Australia. Zootaxa 4079: 501–533. [CrossRef] [PubMed] [Google Scholar]
- Oliver JD. 1991. Consumption rates, evacuation rates and diets of Pygmy Killifish, Leptolucania ommata, and Mosquitofish, Gambusia affinis in the Okefenokee Swamp. Brimleyana 17: 89–103. [Google Scholar]
- Padhye SM, Dumont HJ. 2014. Moina hemanti sp. nov., a new species of the genus Moina s.l. (Branchiopoda: Anomopoda) from Pune, India. Zootaxa 3860: 561–570. [CrossRef] [PubMed] [Google Scholar]
- Popova EV, Petrusek A, Kořínek V, et al. 2016. Revision of the Old World Daphnia (Ctenodaphnia) similis group (Cladocera: Daphniidae). Zootaxa 4161: 1–40. [CrossRef] [PubMed] [Google Scholar]
- Pulquerio MJ, Nichols RA. 2007. Dates from the molecular clock: how wrong can we be? TREES 22: 180–184. [Google Scholar]
- Purvis A, Gittleman JL, Brooks T. 2005. Phylogeny and conservation. Cambridge University Press, Cambridge, 431 p. [Google Scholar]
- Rammner W. 1937. Beitrag zur Cladocerenfauna von Java. Int Rev Ges Hydrobiol 35: 35–50. [CrossRef] [Google Scholar]
- Rey J, Saint-Jean L. 1969. Les Cladocères (Crustacés, Branchiopodes) du Tchad (Deuxième note). Cahiers ORSTOM série Ser Hydrobiol 3: 21–42. [EDP Sciences] [Google Scholar]
- Richard J. 1892. Sur la distribution géographique des Cladocères. Congrés Int Zool Moscou 2: 1–15. [Google Scholar]
- Sacherová V, Hebert PDN. 2003. The evolutionary history of the Chydoridae (Crustacea: Cladocera). Biol J Linn Soc 79: 629–643. [CrossRef] [Google Scholar]
- Saeng-aroon C. 2001. Species diversity and abundance of Cladocera in Lake Kud-Thing, Nong Khai Province. Ms. Sci. Thesis. Khon Kaen, Thailand: Graduate School in Biology, Khon Kaen University, 105 p. [in Thai] [Google Scholar]
- Saeng-aroon C, Sanoamuang L. 2002. Species diversity and abundance of Cladocera in Lake Kud-Thing, Nong Khai Province. Khon Kaen Univ Res J 7: 14–25 [in Thai]. [Google Scholar]
- Sanoamuang L. 1998. Contributions to the knowledge of the Cladocera of north-east Thailand. Hydrobiologia 362: 45–53. [CrossRef] [Google Scholar]
- Sars GO. 1916. The fresh-water Entomostraca of the Cape Province (Union of South Africa). Part 1: Cladocera. Ann S Afr Mus 15: 303–351. [Google Scholar]
- Schabetsberger R, Drozdowski G, Rott E, et al. 2009. Losing the bounty? Investigating species richness in isolated freshwater ecosystems of Oceania. Pac Sci63: 153–179. [CrossRef] [Google Scholar]
- Schabetsberger R, Kaiser R, Rott E, et al. 2013. On the brink-investigating biodiversity in endangered crater lakes of the Amber Mountains National Park (Madagascar). Aquat Conserv Mar Fresh Ecosyst 23: 316–331. [CrossRef] [Google Scholar]
- Sharma P, Kotov AA. 2013. Molecular approach to identify sibling species of the Ceriodaphnia cornuta complex (Cladocera: Daphniidae) from Australia with notes on the continental endemism of this group. Zootaxa 3702: 79–89. [CrossRef] [PubMed] [Google Scholar]
- Silva-Briano M. 1998. A revision of Macrothricid-like anomopods. Ph.D. Thesis. Ghent University, 388 p. [EDP Sciences] [Google Scholar]
- Silva-Briano M, Dieu NQ, Dumont HJ. 1999. Redescription of Macrothrix laticornis (Jurine, 1820), and description of two new species of the M. laticornis-group. Hydrobiologia 403: 39–61. [CrossRef] [Google Scholar]
- Sinev AY. 1997. Review of the affinis-group of Alona Baird, 1843, with the description of a new species from Australia (Anomopoda Chydoridae). Arthropoda Selecta 6: 47–58. [Google Scholar]
- Sinev AY. 2004. Miralona gen. n. – a new genus of the subfamily Aloninae (Anomopoda, Chydoridae) from Australia. Hydrobiologia 526: 3–14. [CrossRef] [Google Scholar]
- Sinev AY. 2006. Alona meridionalis sp.n. – a new species of Chydoridae (Branchiopoda: Cladocera: Anomopoda) from South Africa, with transverse lateral head pores. Arthropoda Selecta 15: 193–202. [Google Scholar]
- Sinev AY. 2008. A new species related to Alona costata Sars, 1862 (Cladocera: Anomopoda: Chydoridae) from South Africa. Zootaxa 1707: 23–36. [Google Scholar]
- Sinev AY, Hollwedel W. 2002. Alona brandorffi sp.n. (Crustacea: Anomopoda: Chydoridae) – a new species from Brazil, related to A. verrucosa Sars, 1901. Hydrobiologia 472: 131–140. [CrossRef] [Google Scholar]
- Sinev AY, Kotov AA. 2012. New and rare Aloninae (Cladocera: Anomopoda: Chydoridae) from Indochina. Zootaxa 3334: 1–28. [Google Scholar]
- Sinev AY, Sanoamuang L. 2007. Alona siamensis sp.n., a new species of Cladocera from South-East Asia, related to Alona dentifera (Sars, 1901) (Anomopoda: Chydoridae). Arthropoda Selecta 16(3): 143–150. [Google Scholar]
- Sinev AY, Shiel RJ. 2012. Extremalona timmsi gen. nov., sp. nov., a new cladoceran (Cladocera: Anomopoda: Chydoridae) from an acid saline lake in southwest Western Australia. J Nat Hist 46: 2845–2864. [CrossRef] [Google Scholar]
- Sinev AY, Kotov AA, Van Damme K. 2004. Morphology of a Neotropical cladoceran Alona dentifera (Sars, 1901), and its position within the family Chydoridae Stebbing, 1902 (Branchiopoda: Anomopoda). Arthropoda Selecta 13: 99–107. [EDP Sciences] [Google Scholar]
- Sinev AY, Van Damme K, Kotov AA. 2005. Redescription of tropical-temperate cladocerans Alona diaphana King, 1853 and Alona davidi Richard, 1895 and their translocation to LeberisSmirnov, 1989 (Branchiopoda: Anomopoda: Chydoridae). Arthropoda Selecta 14: 183–205. [Google Scholar]
- Sinev AY, Garibian PG, Gu Y. 2016. A new species of Pseudochydorus Fryer, 1968 (Cladocera: Anomopoda: Chydoridae) from South-East Asia. Zootaxa4079: 129–139. [CrossRef] [PubMed] [Google Scholar]
- Smirnov NN. 1976. Macrothricidae and Moinidae of the World fauna. Fauna SSSR, novaya seriya. Rakoobraznye 1: 1–237 [in Russian] [Google Scholar]
- Smirnov NN. 1992. The Macrothricidae of the world. Guides to the identification of the microivertebrates of the Continental Waters of the world, vol 1. The Hague: SPB Academic Publishing, pp. 1–143. [Google Scholar]
- Smirnov NN. 1995. Check-list of the Australian Cladocera (Crustacea). Arthropoda Selecta 4: 3–6. [Google Scholar]
- Smirnov NN. 1996. Cladocera: the Chydorinae and Sayciinae (Chydoridae) of the world. Guides to the identification of the microivertebrates of the Continental Waters of the world, vol 11. Amsterdam: SPB Academic Publishing, pp. 1–197. [Google Scholar]
- Smirnov NN. 2008. Check-list of the South-African Cladocera (Crustacea: Branchiopoda). Zootaxa 1788: 47–56. [Google Scholar]
- Smirnov NN, Bayly IAE. 1995. New records and further description of Macrothrix hardingi Petkovski (Cladocera) from granite pools in Western Australia. J R Soc West Austr 78: 13–14. [Google Scholar]
- Smirnov NN, Timms BV. 1983. A revision of the Australian Cladocera (Crustacea). Rec Aust Mus Suppl 1: 1–132. [CrossRef] [Google Scholar]
- Smirnov NN, Timms BV. 1984. Cladocera (Crustacea) of Australia: Fauna composition and zoogeography. Zool Zh 63: 1792–1796. [Google Scholar]
- Smith GW. 1909. The freshwater Crustacea of Tasmania, with remarks on their geographical distribution. Trans Linn Soc Lond Ser 2 (Zool) 11: 61–92. [CrossRef] [Google Scholar]
- Sousa FDR, Santos S, Güntzel AM, et al. 2015. Description of a new species of the costata-group (Cladocera, Chydoridae, Aloninae) from Brazil. Zootaxa 4040: 445–457. [CrossRef] [PubMed] [Google Scholar]
- Sousa FDR, Elmoor-Loureiro LMA, Santos S. 2016a. New findings of Hexalona-branch representatives in Brazil, with a description of Prenda gen. nov. (Crustacea: Anomopoda: Aloninae). J Nat Hist 50: 2727–2768. [CrossRef] [Google Scholar]
- Sousa FDR, Elmoor-Loureiro LMA, Santos S. 2016b. Position of the dentifera-group in the Coronatella-branch and its relocation to a new genus: Magnospina gen. n. (Crustacea, Chydoridae, Aloninae). ZooKeys 586: 95–119. [CrossRef] [Google Scholar]
- Swofford D. 1993. PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods). Version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Tanaka S, Ohtaka A. 2010. Freshwater Cladocera (Crustacea, Branchiopoda) in Lake Tonle Sap and its adjacent waters in Cambodia. Limnology 11: 171–178. [CrossRef] [Google Scholar]
- Thomas IF. 1961. The Cladocera of the swamps of Uganda. Crustaceana 2: 108–125. [CrossRef] [Google Scholar]
- Van Damme K. 2016. Endemism and long distance dispersal in the waterfleas of Easter Island. Zootaxa 4154: 251–272. [CrossRef] [PubMed] [Google Scholar]
- Van Damme K, Dumont HJ. 2009. Notes on chydorid endemism in continental Africa: Matralona gen. n., a monotypic Alonine from the Fouta Djalon Plateau (Guinea, West Africa) (Crustacea: Cladocera: Anomopoda). Zootaxa 2051: 26–40. [Google Scholar]
- Van Damme K, Dumont HJ. 2010 Cladocera of the Lençóis Maranhenses (NE-Brazil): faunal composition and a reappraisal of Sars' Method. Braz J Biol 70: 755–779. [CrossRef] [Google Scholar]
- Van Damme K, Kotov AA. 2016. The fossil record of the Cladocera (Crustacea: Branchiopoda): Evidence and hypotheses. Earth Sci Rev 163: 162–189. [CrossRef] [Google Scholar]
- Van Damme K, Maiphae S. 2013. Salinalona gen. nov., an euryhaline chydorid lineage (Crustacea: Branchiopoda: Cladocera: Anomopoda) from the Oriental region. J Limnol 72: 142–173. [Google Scholar]
- Van Damme K, Sinev AY. 2013. Tropical Amphi-Pacific disjunctions in the Cladocera (Crustacea: Branchiopoda). J Limnol 72: 209–244. [Google Scholar]
- Van Damme K, Shiel RJ, Dumont HJ. 2007. Notothrix halsei gen. n., sp. n., representative of a new family of freshwater cladocerans (Branchiopoda, Anomopoda) from SW Australia, with a discussion of ancestral traits and a preliminary molecular phylogeny of the order. Zool Scr 36: 465–487. [CrossRef] [Google Scholar]
- Van Damme K, Sinev AY, Dumont HJ. 2011. Separation of Anthalona gen.n. from Alona Baird, 1843 (Branchiopoda: Cladocera: Anomopoda): morphology and evolution of scraping stenothermic alonines. Zootaxa 2875: 1–64. [Google Scholar]
- Van Damme K, Bekker EI, Kotov AA. 2013a. Endemism in the Cladocera (Crustacea: Branchiopoda) of Southern Africa. J Limnol 72: 440–463. [Google Scholar]
- Van Damme K, Maiphae S, Sa-ardrit P. 2013b. Inland swamps in South East Asia harbour hidden cladoceran diversities: species richness and the description of new paludal Chydoridae (Crustacea: Branchiopoda: Cladocera) from Southern Thailand. J Limnol 72: 174–208. [Google Scholar]
Cite this article as: Neretina AN, Kotov AA. 2017. Diversity and distribution of the Macrothrix paulensis species group (Crustacea: Cladocera: Macrothricidae) in the tropics: what can we learn from the morphological data? Ann. Limnol. - Int. J. Lim. 53: 425–465
All Tables
Comparison between Macrothrix paulensis-like species (based on original data and Sars, 1916; Harding, 1955; Idris and Fernando, 1981b; Ciros-Pérez and Elías-Gutiérrez, 1997; Dumont et al., 2002; Kotov and Hollwedel, 2004; Kotov et al., 2005, 2010; Garfias-Espejo et al., 2007).
Comparison between Australian members of the genus Macrothrix Baird, 1843 (after Smith, 1909; Gurney, 1927; Smirnov and Timms, 1983; Smirnov, 1976 (we kept Russian letters for original illustrations); 1992 and our current data)
Data matrix of 19-morphological characters in 12 taxa used in cladistic analysis. Data missing, or varying - .
All Figures
 |
Fig. 1 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Adult parthenogenetic female, general view. B, Head. C, Labrum. D, Armature of anterior margin of valve. E, Armature of ventral margin of valve. F, Armature of posterior margin of valve. G, Postabdomen. H, Distal portion of postabdomen. I, Postabdominal claw, outer view. J, Postabdominal claw, inner view. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 2 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Antenna I. B, Antenna II. C, Lateral seta of basal endopod segment of antenna II. D, Its central part. E, Its distal part. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 3 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Head (ventral view). B, Dorsal pore. C, Central part of valve. D, Postabdomen. E, Distal portion of postabdomen. F–G, Antenna I. H, Antenna II. I, Exopod and endopod branches of antenna II. Scale bars: 0.2 mm for D, H, 0.1 mm for A, F–G, I, 0.05 mm for C, E, 0.01 mm for B. |
| In the text | |
 |
Fig. 4 Macrothrix capensis (Sars, 1916), parthenogenetic female from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Corm of limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. F, Limb IV. G, Limb V. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 5 Macrothrix capensis (Sars, 1916), females from Rocher Pan (S 32.6094°, E 18.3003°), Western Cape, coll. 11.12.2000 by G. Jones, NNS-2002-244. A, Parthenogenetic female. B–E, Ephippial female. A, Central part of lateral seta of basal endopod segment of antenna II. B, Ephippium (general view). C, Distal segments of antennal branches. D, Apical swimming setae. E, Fragment of ephippium. Scale bars: 0.2 mm for B, 0.1 mm for C, 0.02 mm for A, D–E. |
| In the text | |
 |
Fig. 6 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Lake Kud-Thing in floodplain of Mekong River, Nong Khai Province, Thailand, coll. 28.11.1998 by C. Saeng-aroon, AAK-2003-033. A, General view. B, Head. C, Armature of ventral margin of valve. D, Antenna I. E, Antenna II. F, Exopod and endopod branches of antenna II. G, Central part of lateral seta of basal endopod segment of antenna II. Scale bars: 0.2 mm for A, E, 0.1 mm for B, D, 0.05 mm for C, F, 0.2 mm for G. |
| In the text | |
 |
Fig. 7 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, General view. B, Head. C, Labrum. D, Valve. E, Armature of anterior margin of valve. F, Armature of ventral margin of valve. G, Armature of posterior margin of valve. H, Postabdomen. I, Distal portion of postabdomen. J, Postabdominal seta. K, Postabdominal claw, outer view. L, Postabdominal claw, inner view. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 8 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Antenna I. B, Distal portion of antenna I. C, Antenna II. D, Central part of lateral seta of basal endopod segment of antenna II. E, Central part of lateral seta of middle endopod segment of antenna II. F, Central part of lateral exopod segment seta. G–H, Apical swimming setae in different position. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 9 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A–B, Armature of ventral margin of valve. C, Central part of valve. D, Postabdomen. E–F, Distal portion of postabdomen. G, Postabdominal claws. H, Antenna I. Scale bars: 0.2 mm for D, 0.05 mm for A, E–F, H, 0.02 mm for B–C, G. |
| In the text | |
 |
Fig. 10 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool, “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Antenna II. B, Exopod and endopod branches of antenna II. C–D, Proximal portion of lateral seta of basal endopod segment of antenna II. E–G, Central part of lateral seta of basal endopod segment of antenna II. Scale bars: 0.2 mm for A, 0.1 mm for B, 0.05 mm for E, 0.02 mm for C–D, F–G. |
| In the text | |
 |
Fig. 11 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool, “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Corm of limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 12 Macrothrix odiosa Gurney, 1916, parthenogenetic female from a pool, “Yizeb” before Hamusit (N 11.7333°, E 37.5166°), Ethiopia, coll. 24.09.2015 by W. Zelalem, ANN-2016-001. A, Limb IV. B, Fragment of limb IV. C, Limb V. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 13 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, General view. B, Head (lateral view). C, Head (ventral view). D, Labrum. E, Valve. F, Armature of anterior margin of valve. G, Armature of ventral margin of valve. H, Armature of posterior margin of valve. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 14 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, Postabdomen. B, Distal portion of postabdomen. C, Distal segment of postabdominal seta. D, Postabdominal claw, outer view. E, Postabdominal claw, inner view. F, Antenna I. G, Antenna II. H, Middle potion of lateral seta of basal endopod segment of antenna II. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 15 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, General view. B, Head. C, Armature of ventral margin of valve. D, Central part of valve. E, Postabdomen. F, Postabdominal claw. G, Antenna II. H–I, Central part of lateral seta of basal endopod segment of antenna II. J, Apical swimming setae of antenna II. K, Mandible. L, Limb I. Scale bars: 0.5 mm for A, 0.2 mm for B, G 0.1 mm for E, 0.05 mm for C–D, J, L, 0.02 mm for F, H–I, K. |
| In the text | |
 |
Fig. 16 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, Corm of limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 17 Macrothrix odiosa Gurney, 1916, parthenogenetic female from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, Limb IV. B, Fragment of limb IV. C, Limb V. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 18 Macrothrix odiosa Gurney, 1916 from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A–C, Ephippial female. D–E, Adult male. A, Ephippial female, general view. B, Ephippium, general view. C, Fragment of ephippium. D, Male, general view. E, Male, Head. Scale bars: 0.2 mm for A–B, D, 0.1 mm for E, 0.02 mm for C. |
| In the text | |
 |
Fig. 19 Macrothrix odiosa Gurney, 1916, adult male from Glen Avis rock pool 4 (S 30.8053°, E 28.2214°), McClear, Eastern Cape, the Republic of South Africa, coll. 29.03.1993 by K. Martens, de Moor and Barber, NNS-2002-131. A, General view. B, Head. C, Postabdomen. D, Antenna I. E, Fragment of limb I. F, Male hook. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 20 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, General view. B, Head. C, Dorsal pore. D, Labrum. E, Valve. F, Armature of anterior margin of valve. G, Armature of ventral margin of valve. H, Armature of posterior margin of valve. I, Postabdomen. J, Distal portion of postabdomen. K, Postabdominal claw, outer view. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 21 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Antenna I. B, Antenna II. C, Lateral seta of basal endopod segment of antenna II. D, Its central part. E, Its distal part. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 22 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Valve. B, Dorsal pore. C, Armature of ventral margin of valve. D, Central part of valve. E, Postabdomen. F, Postabdominal claw. G–H, Exopod and endopod branches of antenna II. I, Central part of lateral seta of basal endopod segment of antenna II. J, Swimming setae of antenna II. K, Apical spines of antenna II. Scale bars: 0.5 mm for A, 0.1 mm for E, J–K, 0.05 mm for D, H, 0.02 mm for F, 0.01 mm for B–C, G, I. |
| In the text | |
 |
Fig. 23 Macrothrix australiensis sp. nov., parthenogenetic female from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Limb I. B, Distal part of limb I. C, Limb II. D, Limb III. E, Fragment of limb III. F, Limb IV. G, Limb V. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 24 Macrothrix australiensis sp. nov., from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, Parthenogenetic female, ventral view (small spines on antenna I are marked via arrow). B–C, Ephippial female. D, Postabdomen of adult male (gonopores is marked via arrow). Scale bars: 0.1 mm for A, 0.2 mm for B–C, 0.02 for D. |
| In the text | |
 |
Fig. 25 Macrothrix australiensis sp. nov., adult male from Lake Fox (individuals from culture of A.V. Makrushin), South Australia, collection details unknown, AAK-2005-197. A, General view. B, Head. C, Postabdomen. D, Antenna I. E, Limb I. Scale bars: 0.1 mm. |
| In the text | |
 |
Fig. 26 A strict consensus of 18 equally-parsimonious trees for all investigated members of Macrothrix paulensis species group and map of their distribution (TL = 31, CI = 0.807, RI = 0.842). The 50% majority rule bootstrap simulation led a tree of similar topology with the contree. Due to this fact, branch probabilities were assigned to the aforementioned contree. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


