| Issue |
Int. J. Lim.
Volume 60, 2024
|
|
|---|---|---|
| Article Number | 22 | |
| Number of page(s) | 7 | |
| DOI | https://doi.org/10.1051/limn/2024022 | |
| Published online | 30 October 2024 | |
Short Note
Artificial floating littoral zones: a promising nursery to support Pike (Esox lucius) in reservoirs
1
INRAE, Aix Marseille Univ, RECOVER, 3275 Route Cézanne, 13100 Aix-en-Provence, France
2
OFB, Service ECOAQUA, DRAS, 3275 Route Cézanne, 13100 Aix-en-Provence, France
3
Pôle R&D Ecosystèmes Lacustres (ECLA), OFB-INRAE-USMB, Aix-en-Provence, France
4
ECOCEAN, 1342 Avenue de Toulouse, 34070 Montpellier, France
* Corresponding author: quentin.salmon@inrae.fr; samuel.westrelin@inrae.fr
Received:
29
February
2024
Accepted:
23
September
2024
The use of water resources in reservoirs leads to artificial water level fluctuations sometimes with extreme amplitudes and frequencies. These artificial fluctuations homogenize littoral habitats and often make macrophytes disappear. Consequently, spawning and refuge-nursery habitats become scarce which is critical for phytophilous species such as Northern Pike (Esox lucius) whose populations decline. Quite recently, floating artificial structures have emerged as a mitigation solution. However, the design of these structures is relatively simplistic and only consists in a simple 2D-floating mat of vegetation. Their effectiveness to support fish populations, especially pike in regulated reservoir, by providing suitable habitats for spawning, refuge and nursery remains poorly documented. Here we conceived 3D artificial Floating Littoral Zones (FLOLIZ) that mimic a natural littoral zone to support both pike spawning and juvenile growth (helophytes, hydrophytes, specific shelter areas). To assess their effectiveness, three structures of 70 m2 area and 1 m deep were installed in September 2018 in a French reservoir subject to extreme water level fluctuations. Visual surveys carried out in spring and summer by snorkelling over 2019–2023 highlighted an annual recurrence of juvenile pike in FLOLIZ, but also a higher abundance than in control stations. The maximum number of individuals observed simultaneously on the same structure was 14. Even if it cannot be asserted that pike spawned in FLOLIZ, these results highlight that FLOLIZ can provide refuge and nursery habitats for juvenile fish in reservoirs with poor littoral habitats.
Key words: Ecological engineering / artificial floating island (AFI) / water level fluctuation (WLF) / mitigation / Phytophilous species
© EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Northern Pike (Esox lucius, Linnæus 1758; hereafter “pike”) is widely distributed in the Holarctic region. Pike lives in a variety of freshwater ecosystems and also in brackish ecosystems (Craig, 2008). As an apex predator, it is a keystone species that exerts a top-down control on the structure of fish communities, including its conspecifics (Carpenter et al., 1993; Baum and Worm, 2009). Aquatic vegetation plays a major role during the life cycle of pike for spawning, sheltering and foraging (Bry, 1996), and particularly for juvenile stages (Craig, 2008; Skov and Nilsson, 2018). The reproductive success of pike is highly dependent on both the density and type of vegetation available and the stability of the water level at key periods (Casselman and Lewis, 1996). Specifically, the water level must be stable for at least four weeks after egg-laying for the survival and good development of fry (Mingelbier et al., 2008).
In reservoirs, which are used for hydropower and water resource supply, or in regulated rivers, the water level fluctuates artificially. These water level fluctuations (WLF) considerably differ from natural fluctuations in amplitude, duration and frequency (Coops et al., 2003; Hirsch et al., 2014). As a consequence, adjacent wetlands are disconnected and littoral habitats impoverished because they become homogenized with no or very few aquatic vegetation (Furey et al., 2004; Logez et al., 2016; Foubert et al., 2020). This prevents successful spawning and recruitment of pike and leads to an overall decline in their populations (Wolcox and Meeker, 1992; Kruk and Penczak, 2003; Mingelbier et al., 2008; Crane et al., 2015). In France, pike has been classified as “Vulnerable” on the Red List of threatened species (UICN Comité français et al., 2019) and benefits from a biotope protection that prohibits the degradation and destruction of eggs and spawning habitats since 1988. To compensate for low abundances, pike is besides very commonly stocked, what has however been shown not to be very efficient (Monk et al., 2020; Daupagne et al., 2021; Guillerault et al., 2021).
Several mitigation measures of artificial WLF impacts have been listed for reservoirs, such as the diversification of aquatic habitats, bank restoration or even flow management to limit water level variations during sensitive periods (Trussart et al., 2002). However, these mitigation measures can be extremely difficult to implement in the field, remain ineffective with high WLF amplitudes and may also conflict with main uses reservoirs have been created for; in the end, they are implemented in very few reservoirs (Santos et al., 2008, 2011; Baumann et al., 2016). To circumvent these issues, habitats not impacted by WLF such as artificial floating islands appear to be a promising mitigation tool (Nakamura and Mueller, 2008; Yeh et al., 2015; Ware, 2020). Some studies have shown that these floating structures were attractive for fish (Hwa-Keun Byeon, 2014; Huang et al., 2017), especially for phytophilous species such as pike (Gillet, 1989; De Moraes et al., 2023), but they remain scarce. Furthermore, the relatively simplistic structures they used (often vegetated 2D floating mat) include habitats suitable for spawning but not for the growth of juvenile fish. Here we conceived a complex 3D floating structure integrating all habitats required for fish and more specifically for pike life cycle, including underwater levels, and tested if it could help to sustain populations in regulated reservoirs.
2 Material and methods
We designed a 70 m2 three-dimensional floating structure (Floating Littoral Zone “FLOLIZ”, 14 m long × 5 m wide) that mimics a natural littoral zone with helophytes, aquatic stages with different depths and vegetated with hydrophytes, and different types of shelter (Fig. 1). In detail, it is composed of a terrestrial part made up of floating caissons (MarineFloor©) and helophyte planters (the roots can grow in water). This floating part supports the frame made up of three extruded aluminium structures (one central structure 0.8 m deep and two edge structures 0.5 m deep). Steel mesh cages containing an inert mineral substrate (recycled oyster shells, Misapore©) cover their bottom and two sides. Grass-like hydrophytes were planted (Stuckenia pectinata, Myriophyllum spicatum, Chara sp) in the 0.5 m deep structures and pondweeds (Potamogeton nodosus, Potamogeton lucens, Potamogeton coloratus) in the 1 m deep structure. Grass-like plants recreated an aquatic lawn that is highly appreciated by pike for egg deposition (Franklin and Smith Jr., 1963; Mccarraher and Thomas, 1972) whereas the dense pondweed beds provided refuge-nursery areas (Cook and Bergersen, 1988; Raat and Nations, 1988; Walton‐Rabideau et al., 2020). Finally, empty wire cages, 2.5 cm mesh size, were placed along the width of the aluminium structures to create shelter for fry. FLOLIZ functionality for pike life cycle (from spawning to growth of juvenile fish) has been tested over 2019-2023 in Serre-Ponçon reservoir (France, 44° 31’ 38.23” N; 6° 22’ 52.04” E, area 28 km2, volume 1270 million m3 and maximum depth 110 m). Over 2019-2022, annual WLF ranged in [17; 49] m and daily WLF in [–1.67; + 1.23] m (Data EDF). Three FLOLIZ have been installed in September 2018 in three downstream bays of the reservoir. For a full description of Serre-Ponçon reservoir and FLOLIZ, refer to Salmon et al. (2022).
Pike were recorded by snorkelling from the beginning of the spawning period (May for Serre-Ponçon reservoir) to the end of summer from 2019 to 2023. This sampling method has already proved its effectiveness for counting pike in lake, especially for age 0+ (Turner and Mackay, 1985; Brosse et al., 2001). In these clear waters, visual census was suitable to detect potential egg-layings in hydrophytes and to record all pike life stages (from larvae to adults) that could be approached closely (<1 m) without escaping. It was conducted by two qualified divers to identify pike, estimate their size and life stage, both in FLOLIZ and in control stations. Control stations were of two kinds: a portion of natural littoral zone in the same bay as FLOLIZ, approximately 50 m away from FLOLIZ (NCS for Nearby Control Stations), and another one in a neighbouring bay with similar hydro morphological characteristics to NCS (DCS for Distant Control Stations). Shallow depth being a main feature of juvenile pike habitat (Chapman and Mackay, 1984), due to steep banks control stations consisted in 70 m long, 1 m wide (i.e. 70 m2) and up to 1 m deep areas. The trio of sampling stations (FLOLIZ, NCS, and DCS) was replicated in three zones of the reservoir (for details, see Salmon et al., 2022). The choice of two kinds of control station aimed to evaluate the influence of FLOLIZ in its surrounding, its attractiveness possibly leading to more pike observations in NCS than in DCS. Each diver sampled half a FLOLIZ in roughly 20 minutes by swimming into and around the FLOLIZ. In control stations, each diver sampled a 35 m-long and 1 m-wide linear in 20 minutes as well. Differences in pike abundance between stations were tested according to different periods: over the entire sampling period 2019–2023, by season (spring corresponds to May–June and summer to July–September) and by year (2019, 2020, 2021, 2022 and 2023). Non-parametric two-by-two comparisons were used to highlight differences in abundance. Tests were performed using the “kruskal.test” function in the « Stats » package (R Core Team, 2022). The analysis was carried out in Rstudio software version 2022.12.0-353.
 |
Fig. 1 The different parts of the FLOLIZ. (A) Floating part with helophytes; (B) Aquatic part with wire cages; (C) Helophyte roots and (D) Pondweed bed. Author: Remy DUBAS. ©UROS Project (OFB-INRAE-ECOCEAN). |
3 Results
No egg laying was observed neither in FLOLIZ nor in control stations during the sampling period. A total of 43 observations of pike were recorded among which 39 corresponded to juveniles and 4 to adults (Fig. 2). In FLOLIZ, pike were observed in each year totalling 29 observations (2 adults and 27 juveniles). For the control stations, only 3 observations (1 adult and 2 juveniles) in the DCS and 11 observations (1 adult and 10 juveniles) in the NCS were made over the sampling period. The mean abundance of pike was significantly higher in FLOLIZ than in DCS over the whole sampling period (0.6 ± 2.2 in FLOLIZ and 0.07 ± 0.3 in DCS, P-value = 0.049) and also in spring (1.4 ± 3.3 in the FLOLIZ and 0.1 ± 0.3 in DCS, P-value = 0.037) Table 1. Abundances in NCS and DCS did not differ.
Two adults (total length > 700 mm) were observed in 2022 in two different FLOLIZ, one in May, just after the spawning season. By year, the minimum and maximum numbers of pike recorded were [2; 18] for FLOLIZ, [0; 1] for DCS and [0; 6] for NCS (Fig. 2). The maximum number observed in one station and during one sampling campaign was 14 in FLOLIZ, 5 in NCS and 1 in DCS. Individuals were mainly observed near the surface inside FLOLIZ, either hidden in the roots of helophytes or under floating wood branches (Fig. 3). In control stations, individuals were observed below the surface at a maximum depth of approximately 0.3 m, without any close physical structure. The smallest individuals (∼15 mm-long) observed in FLOLIZ still had yolk sac. One hunting event of pike fry (∼25 mm-long) was observed on cyprinid larvae (∼15 mm-long) in FLOLIZ. The tail of the cyprinid larvae protrudes from the mouth of the juvenile pike (Fig. 3C).
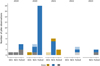 |
Fig. 2 Number of pike individuals observed in the different type of station (DCS, NCS, FLOLIZ) over 2019–2023. The colours correspond to the zone (3 replicates for each station type). The hatched rectangles correspond to adults, while the filled rectangles correspond to juveniles. DCS could not be sampled in 2023. |
Kruskal-Wallis test of differences in pike abundance between station types (FLOLIZ and control stations) over different periods. NS = No significant difference; * means a significant difference at 5% threshold; χ2 = Kruskal-Wallis Chi Squared; df = Degree of freedom; P = P-value (Kruskal and Wallis, 1952).
 |
Fig. 3 Pictures of juvenile pike in FLOLIZ during the sampling period. (A) 15 mm individual (May 2019); (B) 20 mm individual (May 2019); (C) 25 mm individual (May 2019); (D) 70 mm individual (June 2020). Sizes have been estimated visually. The author of photos A to C is Remy DUBAS. The author of photo D is Julien DUBLON. ©UROS Project (OFB-INRAE-ECOCEAN). |
4 Discussion
No previous study had investigated the effectiveness of artificial floating structure to support pike populations in reservoirs with high WLF. This study experimentally tested, for the first time, the attractiveness and effectiveness of a complex floating structure (3D design) as an alternative nursery for pike in reservoirs. From 2019 to 2023, we systematically observed juvenile pike in FLOLIZ but not in littoral control stations. Over the whole sampling period, a higher pike abundance was observed in FLOLIZ than in DCS, but not than in NCS; however, this was not verified in each year. This could strengthen a possible influence of FLOLIZ beyond its own area and illustrate its ability to support the population. The attractiveness of FLOLIZ could be related to the complexity and diversity of habitats they provide. Indeed, many studies have shown that early fish stages are highly dependent on complex habitats such as aquatic vegetation (Rozas and Odum, 1988; Grenouillet et al., 2002; Weber and Brown, 2012; Massicotte et al., 2015). Casselman and Lewis (1996) indicate that spawning habitats are less critical than macrophyte cover, which is more critical as a nursery habitat for juvenile pike. In particular, the aquatic vegetation offers to juvenile pike shelter from predators (Skov and Berg, 1999), including conspecifics (Craig, 2008). It also provides places to ambush zooplankton, macroinvertebrates and other fish (Nilsson et al., 2023). In addition, food resources were available in FLOLIZ, with both a high abundance of macroinvertebrates (Salmon et al., 2022) and cyprinid fry (Field Data). These refuge-nursery habitats enabled juvenile pike to grow, from roughly ∼20 mm to ∼60 mm from early May to end of June (Figs. 3A–3C), what enhances their chances of survival and recruitment (Rozas and Odum, 1988). As pike has been shown to exhibit natal homing and fidelity to spawning site (Miller et al., 2001; Larsson et al., 2015), individuals that have grown in FLOLIZ might return in FLOLIZ to spawn once they become mature. Although no egg laying was recorded, the observation of fixed pike larvae with yolk sac as well as mature adults makes very plausible that these larvae hatched in the FLOLIZ. Pike eggs are small (1.5 mm to 3 mm diameter), scattered in dense vegetation (Murry et al., 2008), and hatch in about 10 days at this period (120 °C-days from Frost and Kipling, 1967), what makes them difficult to observe. Due to the complex habitats in FLOLIZ in which juvenile pike can easily hide, we can also reasonably suppose that the number of individuals has been underestimated in comparison to control stations. As regards to the limited size of FLOLIZ compared to space requirements of pike spawning grounds (1500 m2 according to Chancerel, 2003) and home range (Cook and Bergersen, 1988; Sandlund et al., 2016) given in literature, they could probably host only a few adults and would be more adapted to sustain populations in small artificial lakes. Even though FLOLIZ area is relatively small, which could limit the number of spawners they can host, they offer suitable habitats for larvae and juvenile fish (up to 14 individuals observed simultaneously in a FLOLIZ). This could enhance recruitment that has been shown to be much more efficient than restocking to support populations (Skov et al., 2011; Hühn et al., 2014; Hühn et al., 2023). Finally, even if further experiments in different contexts are needed, these results obtained over a five-year period give a promising insight in the effectiveness of FLOLIZ to provide functional refuge and nursery habitats for pike, which could have positive effects on populations in reservoirs or artificial lakes.
Acknowledgements
This project was carried out by the French Biodiversity Agency (OFB), the French National Research Institute for Agriculture, Food and the Environment (INRAE), ECOCEAN Company and the French National Agency for Research and Technology (ANRT). The authors are very grateful to the managers of Serre-Ponçon reservoir (SMADESEP « Syndicat Mixte d'Aménagement et de Développement du Lac de Serre-Ponçon ») for hosting this project and for their great collaboration. Thanks to the “Conservatoire Botanique National Alpin” (CBNA) for their help in planting the structures. Many thanks to local organizations for their enthusiasm in the project: Commission de Serre-Ponçon; EDF “Electricité de France”; Fishing federation of Hautes-Alpes and Alpes-de-Haute-Provence; DDT Direction Départementale des Territoires des Hautes-Alpes et des Alpes-de-Haute-Provence. Thanks to EDF for sharing the data on water level fluctuations. Many thanks to Remy DUBAS (ECOCEAN) for his high-quality pictures.
References
- Baum JK, Worm B. 2009. Cascading top-down effects of changing oceanic predator abundances. J Anim Ecol 78: 699–714. [CrossRef] [PubMed] [Google Scholar]
- Baumann JR, Oakley NC, McRae BJ. 2016. Evaluating the effectiveness of artificial fish habitat designs in turbid reservoirs using sonar imagery. North Am J Fish Manag 36: 1437–1444. [CrossRef] [Google Scholar]
- Brosse S, Laffaille P, Gabas S, Lek S. 2001. Is scuba sampling a relevant method to study fish microhabitat in lakes? Examples and comparisons for three European species. Ecol Freshw Fish 10: 138–146. [CrossRef] [Google Scholar]
- Bry C. 1996. Role of vegetation in the life cycle of pike, in: Pike: Biology and Exploitation. Springer, pp. 45–67. [Google Scholar]
- Carpenter SR, Kitchell JF, Hodgson JR. 1993. Cascading trophic interactions. Trophic Cascade in Lakes 1–14. [Google Scholar]
- Casselman JM, Lewis CA. 1996. Habitat requirements of northern pike (Essox lucius). Can J Fish Aquat Sci 53: 161–174. [CrossRef] [Google Scholar]
- Chapman CA, Mackay WC. 1984. Versatility in habitat use by a top aquatic predator, Esox lucius L. J Fish Biol 25: 109–115. [Google Scholar]
- Cook MF, Bergersen EP. 1988. Movements, habitat selection, and activity periods of northern pike in eleven mile reservoir, Colorado. Trans Am Fish Soc 117: 495–502. [CrossRef] [Google Scholar]
- Coops H, Beklioglu M, Crisman TL. 2003. The role of water-level fluctuations in shallow lake ecosystems − workshop conclusions. Hydrobiologia 506–509: 23–27. [CrossRef] [Google Scholar]
- Craig JF. 2008. A short review of pike ecology. Hydrobiologia 601: 5–16. [CrossRef] [Google Scholar]
- Crane DP, Miller LM, Diana JS, Casselman JM, Farrell JM, Kapuscinski KL, Nohner JK. 2015. Muskellunge and Northern Pike ecology and management: important issues and research needs. Fisheries 40: 258–267. [CrossRef] [Google Scholar]
- Daupagne L, Rolan‐Meynard M, Logez M, Argillier C. 2021. Effects of fish stocking and fishing pressure on fish community structures in French lakes. Fish Manag Ecol 28: 317–327. [CrossRef] [Google Scholar]
- De Moraes KR, Souza AT, Muška M, Hladík M, Čtvrtlíková M, Draštík V, Kolařík T, Kučerová A, Krolová M, Sajdlová Z, Šmejkal M, Kubečka J. 2023. Artificial floating islands: a promising tool to support juvenile fish in lacustrine systems. Hydrobiologia 850: 1969–1984. [CrossRef] [Google Scholar]
- Foubert A, Lecomte F, Brodeur P, Le Pichon C, Mingelbier M. 2020. How intensive agricultural practices and flow regulation are threatening fish spawning habitats and their connectivity in the St. Lawrence River floodplain, Canada. Landscape Ecol 35: 1229–1247. [Google Scholar]
- Franklin DR, Smith Jr., LL. 1963. Early life history of the Northern Pike, Esox lucius L., with special reference to the factors influencing the numerical strength of year classes.Trans Am Fish Soc 92: 91–110. [CrossRef] [Google Scholar]
- Frost WE, Kipling C. 1967. A study of reproduction, early life, weight-length relationship and growth of pike, Esox lucius L., in windermere. J Anim Ecol 651–693. [Google Scholar]
- Furey PC, Nordin RN, Mazumder A. 2004. Water level drawdown affects physical and biogeochemical properties of littoral sediments of a reservoir and a natural lake. Lake Reservoir Manag 20: 280–295. [CrossRef] [Google Scholar]
- Gillet C. 1989. Réalisation de frayères artificielles flottantespour les poissons lacustres. Hydroécol Appl 1: 145–193. [CrossRef] [EDP Sciences] [Google Scholar]
- Grenouillet G, Pont D, Seip KL. 2002. Abundance and species richness as a function of food resources and vegetation structure: juvenile fish assemblages in rivers. Ecography 25: 641–650. [CrossRef] [Google Scholar]
- Guillerault N, Loot G, Blanchet S, Millet P, Musseau C, Cucherousset J, Santoul F. 2021. Efficiency of Northern pike (Esox lucius) stocking in metropolitan France at large spatial and temporal scales. Fish Manag Ecol 28: 486–495. [CrossRef] [Google Scholar]
- Hirsch PE, Schillinger S, Weigt H, Burkhardt-Holm P. 2014. A hydro-economic model for water level fluctuations: combining limnology with economics for sustainable development of hydropower. PLoS ONE 9: e 114889. [Google Scholar]
- Huang X, Zhao F, Song C, Gao Y, Geng Z, Zhuang P. 2017. Effects of stereoscopic artificial floating wetlands on nekton abundance and biomass in the Yangtze Estuary. Chemosphere 183: 510–518. [CrossRef] [PubMed] [Google Scholar]
- Hühn D, Gwinn DC, Shaw SL, Alós J, Allen MS, Pagel T, Skov C, Arlinghaus R. 2023. Density- and size-dependent mechanisms modulate the outcome of stocking in a naturally recruiting freshwater piscivore (northern pike, Esox lucius): a replicated whole-lake experiment. Fish Res 267: 106799. [CrossRef] [Google Scholar]
- Hühn D, Lübke K, Skov C, Arlinghaus R. 2014. Natural recruitment, density-dependent juvenile survival, and the potential for additive effects of stock enhancement: an experimental evaluation of stocking northern pike (Esox lucius) fry. Can J Fish Aquat Sci 71: 1508–1519. [CrossRef] [Google Scholar]
- Hwa-Keun Byeon. 2014. Fish and efficiency on attached fish eggs of artificial floating island in Lake Soyang, Korea. Korean J Environ Ecol 28: 559–565. [CrossRef] [Google Scholar]
- Kruk A, Penczak T. 2003. Impoundment impact on populations of facultative riverine fish. Ann Limnol − Int J Lim 39: 197–210. [CrossRef] [EDP Sciences] [Google Scholar]
- Kruskal WH, Wallis WA. 1952. Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47: 583–621. [CrossRef] [Google Scholar]
- Larsson P, Tibblin P, Koch-Schmidt P, Engstedt O, Nilsson J, Nordahl O, Forsman A. 2015. Ecology, evolution, and management strategies of northern pike populations in the Baltic Sea. AMBIO 44: 451–461. [CrossRef] [PubMed] [Google Scholar]
- Linnæus C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. pp. [1–4], 1–824. Holmiæ. (Salvius). [Google Scholar]
- Logez M, Roy R, Tissot L, Argillier C. 2016. Effects of water-level fluctuations on the environmental characteristics and fish-environment relationships in the littoral zone of a reservoir. Fal 189: 37–49. [CrossRef] [Google Scholar]
- Massicotte P, Bertolo A, Brodeur P, Hudon C, Mingelbier M, Magnan P. 2015. Influence of the aquatic vegetation landscape on larval fish abundance. J Great Lakes Res 41: 873–880. [CrossRef] [Google Scholar]
- Mccarraher DB, Thomas RE. 1972. Ecological significance of vegetation to Northern Pike, Esox lucius, spawning. Trans Am Fish Soc 101: 560–563. [Google Scholar]
- Miller LM, Kallemeyn L, Senanan W. 2001. Spawning-site and natal-site fidelity by Northern pike in a large lake: Mark-recapture and genetic evidence. Trans Am Fish Soc 130: 307–316. [CrossRef] [Google Scholar]
- Mingelbier M, Brodeur P, Morin J. 2008. Spatially explicit model predicting the spawning habitat and early stage mortality of Northern pike (Esox lucius) in a large system: the St. Lawrence River between 1960 and 2000. Hydrobiologia 601: 55–69. [CrossRef] [Google Scholar]
- Monk CT, Chéret B, Czapla P, Hühn D, Klefoth T, Eschbach E, Hagemann R, Arlinghaus R. 2020. Behavioural and fitness effects of translocation to a novel environment: whole-lake experiments in two aquatic top predators. J Animal Ecol 89: 2325–2344. [CrossRef] [PubMed] [Google Scholar]
- Murry BA, Farrell JM, Schulz KL, Teece MA. 2008. The effect of egg size and nutrient content on larval performance: implications to protracted spawning in northern pike (Esox lucius Linnæus). Hydrobiologia 601: 71–82. [CrossRef] [Google Scholar]
- Nakamura K, Mueller G. 2008. Review of the Performance of the Artificial Floating Island as a Restoration Tool for Aquatic Environments, in: World Environmental and Water Resources Congress 2008. Presented at the World Environmental and Water Resources Congress 2008, American Society of Civil Engineers, Honolulu, Hawaii, United States, pp. 1–10. [Google Scholar]
- Nilsson PA, Ranåker L, Hulthén K, Nilsson-Örtman V, Brönmark C, Brodersen J. 2023. First-season growth and food of YOY pike (Esox lucius) are habitat specific within a lake. Fisheries Res 259: 106563. [CrossRef] [Google Scholar]
- R Core Team. 2022. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Raat AJP, Nations F, A.O. of the U. 1988. Synopsis of Biological Data on the Northern Pike: Esox Lucius Linnæus, 1758. Food & Agriculture Org. [Google Scholar]
- Rozas LP, Odum WE. 1988. Occupation of submerged aquatic vegetation by fishes: testing the roles of food and refuge. Oecologia 77: 101–106. [CrossRef] [PubMed] [Google Scholar]
- Salmon Q, Colas F, Westrelin S, Dublon J, Baudoin J-M. 2022. Floating Littoral Zone (FLOLIZ): a solution to sustain macroinvertebrate communities in regulated lakes? Ecol Eng 176: 106509. [CrossRef] [Google Scholar]
- Sandlund OT, Museth J, Øistad S. 2016. Migration, growth patterns, and diet of pike (Esox lucius) in a river reservoir and its inflowing river. Fish Res Ecol Fish Lakes Reserv 173: 53–60. [Google Scholar]
- Santos LN, Araújo FG, Brotto DS. 2008. Artificial structures as tools for fish habitat rehabilitation in a neotropical reservoir. Aquatic Conserv 18: 896–908. [CrossRef] [Google Scholar]
- Santos LN, García-Berthou E, Agostinho AA, Latini JD. 2011. Fish colonization of artificial reefs in a large Neotropical reservoir: material type and successional changes. Ecol Appl 21: 251–262. [CrossRef] [PubMed] [Google Scholar]
- Skov C, Nilsson PA. 2018. Biology and ecology of pike. CRC Press. [CrossRef] [Google Scholar]
- Skov C, Berg S. 1999. Utilization of natural and artificial habitats by YOY pike in a biomanipulated lake. Hydrobiologia 408–409: 115–122. [Google Scholar]
- Skov C, Koed A, Baastrup-Spohr L, Arlinghaus R. 2011. Dispersal, growth, and diet of stocked and wild northern pike fry in a shallow natural lake, with implications for the management of stocking programs. North Am J Fish Manag 31: 1177–1186. [CrossRef] [Google Scholar]
- Turner LJ, Mackay WC. 1985. Use of visual sensus for estimating population size in northern pike (Esox lucius). Can J Fish Aquat Sci 42: 1835–1840. [CrossRef] [Google Scholar]
- Trussart S, Messier D, Roquet V, Aki S. 2002. Hydropower projects: a review of most effective mitigation measures. Energy Policy 30: 1251–1259. [CrossRef] [Google Scholar]
- UICN Comité français MNHN, SFI & AFB 2019. La Liste rouge des espèces menacées en France? Chapitre Poissons d’eau douce de France métropolitaine. Paris, France. [Google Scholar]
- Walton‐Rabideau SE, Lédée EJI, Leblanc JP, Szekeres P, Midwood JD, Gallagher AJ, Farrell JM, Cooke SJ. 2020. Spatiotemporal ecology of juvenile Muskellunge (Esox masquinongy) and Northern Pike (Esox lucius) in upper St. Lawrence River nursery bays during their inaugural fall and winter. Ecol Freshw Fish 29: 346–363. [Google Scholar]
- Ware J. 2020. An Assessment of Artificial Floating Islands as a Method of Habitat Creation in Marine Environments. https://doi.org/10.23889/SUthesis.56709. [Google Scholar]
- Weber MJ, Brown ML. 2012. Diel and temporal habitat use of four juvenile fishes in a complex glacial lake. Lake Reservoir Manag 28: 120–129. [CrossRef] [Google Scholar]
- Wolcox DA, Meeker JE. 1992. Implications for faunal habitat related to altered macrophyte structure in regulated lakes in northern Minnesota. Wetlands 12: 192–203. [CrossRef] [Google Scholar]
- Yeh N, Yeh P, Chang Y-H. 2015. Artificial floating islands for environmental improvement. Renew Sustain Energy Rev 47: 616–622. [CrossRef] [Google Scholar]
Cite this article as: Salmon Q, Westrelin S, Dublon J, Abadie E, Baudoin J-M. 2024. Artificial floating littoral zones: a promising nursery to support Pike (Esox lucius) in reservoirs. Int. J. Lim. 60: 22.
All Tables
Kruskal-Wallis test of differences in pike abundance between station types (FLOLIZ and control stations) over different periods. NS = No significant difference; * means a significant difference at 5% threshold; χ2 = Kruskal-Wallis Chi Squared; df = Degree of freedom; P = P-value (Kruskal and Wallis, 1952).
All Figures
 |
Fig. 1 The different parts of the FLOLIZ. (A) Floating part with helophytes; (B) Aquatic part with wire cages; (C) Helophyte roots and (D) Pondweed bed. Author: Remy DUBAS. ©UROS Project (OFB-INRAE-ECOCEAN). |
| In the text | |
 |
Fig. 2 Number of pike individuals observed in the different type of station (DCS, NCS, FLOLIZ) over 2019–2023. The colours correspond to the zone (3 replicates for each station type). The hatched rectangles correspond to adults, while the filled rectangles correspond to juveniles. DCS could not be sampled in 2023. |
| In the text | |
 |
Fig. 3 Pictures of juvenile pike in FLOLIZ during the sampling period. (A) 15 mm individual (May 2019); (B) 20 mm individual (May 2019); (C) 25 mm individual (May 2019); (D) 70 mm individual (June 2020). Sizes have been estimated visually. The author of photos A to C is Remy DUBAS. The author of photo D is Julien DUBLON. ©UROS Project (OFB-INRAE-ECOCEAN). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


