| Issue |
Ann. Limnol. - Int. J. Lim.
Volume 57, 2021
|
|
|---|---|---|
| Article Number | 9 | |
| Number of page(s) | 7 | |
| DOI | https://doi.org/10.1051/limn/2021006 | |
| Published online | 30 March 2021 | |
Research Article
Study of morphotypes and life history of six clones of Lecane bulla (Gosse, 1851) from Quintana Roo, Mexico
1
Centro de Investigación Científica de Yucatán, Unidad de Ciencias del Agua, Cancún, Calle 8, No 39, Mz. 29, Sm 64, C.P. 77500, Quintana Roo, México
2
Centro de Ciencias Básicas, Departamento de Biología, Universidad Autónoma de Aguascalientes, Avenida Universidad 940, Ciudad Universitaria Aguascalientes, Aguascalientes 20131, México
* Corresponding author: jesus.alvarado@cicy.mx
Received:
19
October
2020
Accepted:
19
February
2021
The genus Lecane is highly diverse, there are 209 species, most of which inhabits tropical aquatic systems. In Quintana Roo 42 species have been reported, one of these is Lecane bulla described at the widest distribution throughout the Yucatan peninsula however; their morphotypes and demographic features are unknown. Therefore, the objective of this work was identify the presence of morphotypes L. bulla and their life history traits. We evaluated life history and morphometric data of females and asexual and sexual eggs from the populations were established from clonal strains, which remained in laboratory conditions for 6 months. They were kept in a bioclimatic chamber with photoperiod of 12 hours of light and 12 hours darkness, at a 25 ± 2 °C, and were feeding with the green algae Nannochloropsis oculata at 1 × 106 cell/ml. Thirty-four clonal strains from six locations were analyzed. Statistical analysis determined significant differences between morphometric measurements (p < 0.001) in the six localities as well as showed statistically significant differences in all demographic parameters. In conclusion, this study indicates the possible coexistence in the same geographical area of two different morphotypes of L. bulla, one is a small-sized distributed in the northwest of Quintana Roo and another large-sized in the southwest.
Key words: Clonal culture / invertebrates / species complex / karst aquatic systems
© EDP Sciences, 2021
1 Introduction
The diversity of the rotifers that inhabit aquatic ecosystems is underestimated (Ortells et al., 2000) because phenotypic plasticity might hinder species abundance and challenges to their taxonomy. Phenotypic plasticity refers to an organism's ability to change its morphological structures when changes occur in biotic factors such as food type, density, predation among others and/or abiotic such as temperature, salinity, pH, etc. and when conditions are repositioned these organisms return to their normal structure (Stelzer, 2017).
In consequence, to estimate the diversity of rotifers using morphological information is fundamental, that species undergo morphological variations, intraspecific variation overlaps with interspecific variation (Fontaneto et al., 2005). Morphological characteristics are traditionally observed under the microscope and from discontinuities in the variation of these, it is possible to distinguish between species (Leliaert et al., 2014). Classical morphometry measures identifies morphological changes that occur in organisms based on their linear dimensions. Moreover, it is necessary to identify demographic parameters for each morphotype or strain to understand its population ecology. By developing life tables is possible understand the dynamics of the species (Xi et al., 2013). Because, demographic studies can provide valuable information on the adequacy of environmental conditions (Ma et al., 2010); such as mortality, fertility, survival, average lifespan, generation time, and/or population growth rate (Saucedo-Ríos et al., 2017).
The present study is focus on the genus Lecane one of the most diverse genera of the Monogononta (Segers, 2008). It is expected that this genus has genetic evidence of 13 cryptic species (García-Moralez and Domínguez-Domínguez, 2020). The species L. bulla is one of the 209 species described for the genus, which is considered one of the richest genera in rotifer species (Segers, 2008). Some authors like Koste (1978) classified L. bulla in the genus Monostyla, one of three proposed genera derived from the degree of finger fusion (Koste, 1978). However this character was considered insufficient to establish different genera and so, it was decided to combine these into a single genus Lecane (Sharma, 1978).
As for its distribution L. bulla is widely distributed predominantly in tropical or subtropical environments (Segers, 2008). It is characterized by living mainly in the shallow coastal parts of aquatic ecosystems where they feed on bacteria and detritus (Serranía-Soto, 2003). According to Saucedo-Ríos et al. (2017) they are thermospecific (25 °C), with an average life cycle of seven days and a positive intrinsic growth rate.
As for males, the reports are few due mainly to the rarity of their appearance, which is a consequence of their breeding mechanisms (Segers, 1995). Within the genus, 11 males have been reported including L. bulla (Alvarado-Flores et al., 2017). Males generally have a lagged body, ringed with completely separate fingers (Segers and Rico-Martínez, 2000). It has been proposed that due to these morphological characteristics males can be used as a diagnostic character to delimit species (Sudzuki, 1999).
Therefore, L. bulla is an excellent ecological indicator; common species frequently recorded in the plankton samples collected in the coastal areas of aquatic systems worldwide (Segers and Rico-Martínez, 2000). It is also known that L. bulla is a cosmopolitan species adapted to live in wide environmental conditions, many specialized in a given environment that have high interspecific variability (Saucedo- Ríos et al., 2017).
For example, the study conducted by Walsh et al. (2009) identified three different haplotypes of L. bulla from the Chihuahua desert with a genetic divergence between 12 and 15%. García-Morales and Elías-Gutiérrez (2013) found that L. bulla presented eight different haplotypes distributed in Campeche (1), Mexico City (1), Veracruz (3) and Quintana Roo (3). Finally Moreno et al. (2017) estimated the diversity of rotifer species testing the application of the DNA barcode on resistance eggs in the Mediterranean region. They found 35 operational taxonomic units (OTU), revealing four complex cryptic species, where L. bulla presented two different haplotypes, which affirms the fact that it is a complex of cryptic species. Therefore, the objective of this work is to describe the variability in morphology, and the demographics of the life history of the species of the L. bulla that inhabit the aquatic ecosystems of Quintana Roo.
2 Materials and methods
L. bulla specimens were collected in Quintana Roo, Mexico from August to November 2017. The name of the localities abbreviated, geographical location and their physical and chemical conditions at the time of sampling are presented in Table 1. The populations were established from a single female, which remained in laboratory conditions for 6 months before conducting the experiments. The strains were kept in a photoperiod of 12 h of light and 12 h darkness, at a stable temperature of 25 ± 2 °C, were fed with the green algae Nannochloropsis oculata at 1 × 106 cell/ml, cultivated according to Nichols (1973), adding micro growth medium following the suggestions of AquaFarm®. The EPA medium was prepared by dissolving 96 mg NaHCO4, 60 mg CaSO4, 60 mg MgSO4, and 4 mg KCL, with a pH of 7.3, which was prepared using type I water (ultrapure). The concentration of algal cells was measured with a Neubauer Improved Marienfeld chamber (0.0025 mm2). Life history studies began with 100 asexual eggs. Eggs were observed every 5 h until we collected 12 individuals. The hatching percentages were recorded at 24 h. The neonates were then transferred to the individual wells in a 24-well polystyrene plate (Corning®), with N. oculata at 1 × 106 cell/ml as food and incubated at a temperature of 25 ± 2 °C with a photoperiod of 12:12 light: darkness. The total volume in each well was 950 µl. In total, 72 individuals were observed (12 individuals per location, means 12 replicates per site). After the start of the experiment, in each interval of 12 h we count and eliminate the number of parthenogenetic eggs, the production of male eggs, and the presence of males. For intrinsic growth rate (r) studies we use the same experimental design as life table studies except that, we started with 5 neonates per localities (sites) with a total of 18 replicates (90 neonates per localities, in total 540 neonates analyzed from six localities), during this phase, the production of resistance eggs is counted.
Based on the data collected, we derive the following variables: life expectancy in days (L); age-specific survival (lx), fertility (mx), reproductive value (vx), net reproductive rate (R0), generation time (T), and intrinsic growth rate (r). The following formulas were used the following formula according to Krebs (1985) and Begon et al. (1996):
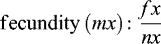
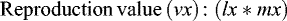

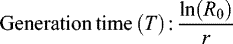 where: x' age structure; nx − number of females alive on a given day; fx − number of offspring/eggs laid on a given day.
where: x' age structure; nx − number of females alive on a given day; fx − number of offspring/eggs laid on a given day.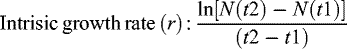 where: Natural Logarithm; No number of individuals; t1: initial time; t2: end time.
where: Natural Logarithm; No number of individuals; t1: initial time; t2: end time.
To analyze the possible effects related to body size with egg size and life table demographics, we measure the body and egg sizes of the six L. bulla populations, measured the length and width of the lorica, and two diameters of parthenogenetic eggs, male eggs and resistance eggs under a composite microscope Axiostar Plus, ZEISS® to a magnification of 20× using the micrometric rule calibrated in the SE64ReL 4.8 software. The volume of the egg (Ve) was calculated from linear measurements using the formula Ve-4/3 × (a2b + ab2)/16, where a and b are the two diameters, assuming that the eggs were general ellipsoids (Ma et al., 2010).
Morphometry measurements were linear. 60 females and 20 males of each strain were randomly selected, in terms of parthenogenetic eggs, resistance and males, only 20 of each were selected by locality. On each photograph, using the micrometric ruler of the AxioVision SE64ReL 4.8 Inc 2003 software, the following sections of each organism were measured: A) length, B) width and C) foot length. The morphometric measurements were analyzed using a variance analysis (ANOVA). We tested the differences in the morphometric measurements within each location, for which a Tukey HSD post hoc test was applied to compare each value (p < 0.05) using R software version 2.6. While for demographic parameters and hatching percentage to determine between which populations there are differences the Duncan Test was used using Statistica 7.0 software. The relationship between body size against demographic parameters and egg volume was analyzed using Pearson's correlation test using Excel's commercial package, Microsoft License.
Environmental data of sampled localities: number of strains; temperature (°C), conductivity (μs/cm), and pH.
3 Results
The ANOVA result for body size was statistically significant since the value of p < 0.001 indicated differences in the size of the L. bulla specimens between the aquatic ecosystems of Quintana Roo. These results indicate that the females from the COR, PC and SCR sites have larger sizes with size range of 120–130 μm in the length of the body, 80–90 μm in the width and feet 65–70 μm in length with respect to the females of the MYL, CHM and PL sites. Females had a body size of 100–110 μm long, with a width of 60–70 μm and a foot length of 45–50 μm ( Tab. 2). The same morphological data occurred in the measurements for the males of the COR, PC and SCR sites. These were larger than the males of MYL, CHM and PL (Tab. 2).
Sixty individuals per laboratory-established strain were analyzed from a total of 34 clonal strains from six locations. Statistical analysis determined significant differences between locality morphometric measurements (ANOVA: asexual females p < 0.00, asexual eggs p < 0.007, unfertilized sexual eggs p > 0.001 and fertilized sexual eggs p < 0.001). Morphometric data of parthenogenetic females and asexual and sexual eggs of L. bulla are presented in Table 2. We test the differences within each locality by comparing the measurements to each other, using a post hoc analysis of Tukey HSD with a p < 0.05. The analysis determined that parthenogenetic females, asexual and sexual eggs from COR, PC, and SCR localities are significantly larger than MYL, PL, and CHM localities (p < 0.00) ( Tab. 3).
Our results about demographic analysis, showed that in six localities there are statistically significant differences in all demographic parameters except for generational time (T) (Tab. 3). Specifically, COR, PC, and SCR locations had a lower life expectancy (L; in days) compared to PL, MYL, and CHM. All localities had an intrinsic growth rate (r) positive, with CHM showing the highest r and lowest COR. The shortest T was SCR (3.7 days) and the longest of MYL (5.4 days); but there were no significant differences. In the case of the net reproductive rate (R0) there were differences among localities, namely if COR, PC and SCR were obtained with MYL, PL and CHM, the previous had a lower reproductive rate compared to the latter. Males were present in all localities; the total number of males that a female can produce throughout its life is presented in Table 3. A female COR can produce up to ten males throughout its life, while a female CHM only produces three.
Age-specific survival (lx) and reproductive value (vx) of the six localities are presented in Figure 1. The behavior of both parameters showed differences between COR, PC, and SCR locations concerning MYL, PL, and CHM locations. The former had the shortest survival, with a reproductive value curve that began from the hatching of females, with a production of up to 2.1 ± 0.1 of parthenogenetic eggs per day, these females continued to contribute reproductive value to the population over time including the day of their death. The latter showed the longest survival, their productive value began between the second and third days of birth, which presents reproductive values of up to 4.3 ± 0.3 parthenogenetic eggs per day, but their production stops 2 days before their death ( Tab. 4).
The hatching percentages of parthenogenetic eggs, male eggs, and the average production of resistance eggs are shown in Table 5. The parthenogenetic eggs of L. bulla were incubated quickly and generally exhibited a success of hatching, except for MYL and PL were less than 50% manage to hatch. For male eggs, their varying hatching rate between COR, PC, and SCR relative to MYL, PL, and CHM, the former had higher hatching percentages compared to seconds were only less than 55% of males came to hatch. In terms of the production of resistance eggs, the locality with the highest number of cysts was COR (12.6 ± 1.6), while CHM had the lowest number with 3.7 ± 0.1 resting egg in total.
Measurements of females and males.
Morphological characterization of individuals of Lecane bulla.
 |
Fig. 1 Survivorship (diamond) and reproductive value (square) of Lecane bulla from six sites in Quintana Roo. COR: Corchalito, PC: Parque del cenote, SCR: El secreto; PL: Punta Laguna, MYL: Muyil, CHM: Chemuyil. X axis days.With a total of 18 replicates. |
Demographic parameters reported for Lecane bulla: lifespan (L; days), instantaneous growth rate of the population (r), generation time (T; days), net reproductive rate (R0) and number of male offspring total (male).
Percentage of asexual eggs and hatched unfertilized eggs after 24 h.
3.1 Size correlation with demographic parameters
Life expectancy (L), generation time (T), net reproductive rate (R0), and intrinsic population rate (r) correlated significantly with females' body size; r2 was 0.90, 0.78, 0.95 and 0.94 ( Fig. 2). That is, it can be observed that females who had the smallest body size have higher demographic parameter values and otherwise with the larger body-sized females had the lowest values. Also, the volume of parthenogenetic eggs, male eggs, and resistance eggs were correlated, with the size of the parthenogenetic females, the values of r2 were 0.97, 0.96 and 0.96, respectively ( Fig. 3). These correlations mean that the volume of the asexual and sexual egg is positively related to the size of the female, that is, small- females will produce small eggs, and large females will produce large eggs.
 |
Fig. 2 Relationship between the demographic parameters (LN) and the body size (length, µm) of the six localities of Lecane bulla of Quintana Roo. N = 20. |
 |
Fig. 3 Relationship between the egg volumen (10 × 4 μm3) and the body size (length, µm) of the six localities of Lecane bulla of Quintana Roo. N = 20. |
4 Discussion
According to Segers and Rico-Martínez (2000), Lecane bulla is one of the most common and frequently recorded species in plankton samples, however little is known about its morphology, as the available information corresponds to specific research related mostly to taxonomic listings (Koste, 2000; Alvarado-Flores et al., 2017). In this study the significant differences between the sizes of the six locations analyzed, indicate the possible coexistence in the same geographical area of two different sizes.
Morphological differentiation does not occur in all rotifer complexes, so it is not always possible to distinguish different morphotypes. For this reason, it is common for several morphotypes to be grouped together in the same species (Kordbacheh et al., 2018). In the L. bulla complex isolated from different aquatic ecosystems in Quintana Roo, this is not the case, since two morphotypes were identified using the analysis of the body size of females and males, as well as the identification of finger, nail and claw structures. The combined use of morphometric measurements with morphological features has been useful to differentiate morphotypes in other complexes of rotifer species such as Brachionus calyciflorus (Mills et al., 2017), B. plicatilis (Michaloudi et al., 2018), Testudinella clypeata (Leasi et al., 2013) or Polyarthra dolichoptera (Obertegger et al., 2014).
L. bulla from COR, PC, and SCR locations distributed in the northwest area of Quintana Roo belong to the largest morphotype. These measurements are consistent with those recorded for L. bulla (Koste, 2000), while strains from MYL, PL, and CHM distributed in the southwest are the smaller morphotypes, which is similar in size to that reported for Sri Lankan L. bulla (Chengalath and Fernando, 1973).
Demographic parameters were shown to have a direct relationship to female size. It was determined that the larger the female's body size, the demographic values tend to be lower, which is consistent with other members of the Lecane genus. The large morphotypes of Lecane species such as: L. papuana, L. quadridentata, and L. luna rang from 120 to 200 μm in the length of the lorica, and have a shorter life expectancy ranging from 5 to 8 days. While small morphotypes of Lecane species such as L. pyriformis, L. tenuiseta and L. cornuta with measurements from 50 to 110 μm in the length of the lorica tend to have a higher expectation ranging from 15 to 26 days (Hummon and Bevelhymer, 1979; Perez-Legaspi and Rico-Martínez, 1998; Serranía-Soto et al., 2011, Saucedo-Rios et al., 2017). The instantaneous rate of population growth (r) is a parameter that represents a population's ability to grow and thrive in an environment (Campillo et al., 2011). In our study, the r values were all positive, indicating population growth but differ with the known ranges of previous studies of other species of the genus Lecane, since those of this work are higher than those reported, including L. bulla from Aguascalientes, Mexico (Saucedo-Ríos et al., 2017).
The percentages of egg hatching are also different among morphotypes. The hatching of parthenogenetic eggs has already been reported in different cryptic species (Gabaldón et al., 2015) but in males, this percentage is poorly known (Xu-Wang et al., 2016). The hatching of resistance eggs was not determined in this study, derived from the difficulties of the species to achieve it (Segers, 1995), but the average production is presented which, as far as the authors know, had not been documented. It has been suggested that the success of hatching is directly related to egg size, i.e. the development of small organisms is faster than that of large individuals (Gillooly et al., 2002). However, this idea does not match the results obtained in this work.
Hatching appears to be related to other factors such as the similarity between haplotypes (Gabaldon et al., 2015), morphological characteristics including body size (Ma et al., 2010), the integrity of eggs in the shell, size of the embryo and the color of eggs (García-Roger et al., 2006) and mainly to life expectancy and survival (Sarma et al., 2017). Females with shorter life cycles had the best percentages of hatching and production. This possibly relates to high rates of asexual reproduction, which leads to rapid population growth, and as a result, females can produce large numbers of parthenogenetic eggs that hatch to remain in the ecosystem. A strategy to compensate for these short periods of life has been observed in rotifers such as L. tenuiseta and L. pyriformis (Hummon and Bevelhymer, 1979).
Age-specific survival curves (lx) and reproductive value (vx), exhibit distinct peaks, large females had present from the first day of hatching, indicating that most animals quickly gained reproductive maturity, it is observed that Lecane species that have a short life cycle, and thus invest their energy in producing eggs in the first 24 hours. While small species do after 48 h.
In conclusion, the estimated diversity of rotifers from Quintana Roo is possibly much higher than previously proposed. The results of this small survey of six populations of the rotifer Lecane bulla from the northern and northwestern region of the Quintana Roo state suggest that. There are morphological differences that allowed for the first time to determine two morphotypes: a large-type morph (G) and small-type morph (P) which is an important finding considering that in many complexes morphological differentiation does not often occur in studies of rotifer diversity worldwide. It is necessary to study the morphological and demographic characteristics, together with genetic differences to establish the total species pool before they are lost due to ecosystem fragmentation or climate change. Also, rotifers like Lecane bulla are excellent ecological indicators that are easy to grow and use in reproductive, physiological, and toxicological tests.
Acknowledgments
Special appreciation to the group of the Lab-Ecotoxicology and for the Ecology Line of Water Science Unit of CICY A.C., in like manner to the groups of research lines on Hydrogeology and Water Quality for their comments and suggestions to the manuscript. The first authors thanks to CONACYT for the scholarship number 803745.
References
- Alvarado-Flores J, Guerrero-Jiménez G, Silva-Briano M, Adabache-Ortíz A, Delgado-Saucedo JJ, Pérez-Yañez D, Marín-Chan A, DeGante-Flores M, Arroyo-Castro JL, Kordbacheh A, Walsh E, Rico-Martínez R. 2017. Sexual reproductive biology of twelve species of rotifers in the genera: Brachionus, Cephalodella, Collotheca, Epiphanes, Filinia, Lecane, and Trichocerca . Mar Freshw Behav Physiol 50: 141–163. [Google Scholar]
- Begon M, Harper JL, Townsend CP. 1996. Ecology: Individuals, Populations, and Communities, 3rd edn. Malden, MA: Blackwell Scientific, 1068 p. [Google Scholar]
- Campillo S, García-Roger EM, Carmona MJ, Serra M. 2011. Local adaptation in rotifer populations. Evol Ecol 25: 933–947. [Google Scholar]
- Chengalath R, Fernando CH. 1973. Rotifera from Sri Lanka (Ceylon) I. The Genus Lecane with description of two new species. Bulletin of the Fisheries Research Station, Sri Lanka (Ceylon) 24: 13–27. [Google Scholar]
- Fontaneto D, Melone G, Ricci C. 2005. Connectivity and nestedness of the metacommunity structure of moss dwelling Bdelloid rotifers along a stream. Hydrobiologia 542: 131–136. [Google Scholar]
- Gabaldón C, Serra M, Carmona MJ, Montero-Pau J. 2015. Life-history traits, abiotic environment and coexistence: the case of two cryptic rotifer species. J Exp Mar Biol Ecol 465: 142–152. [Google Scholar]
- García-Morales AE, Elías-Gutiérrez M. 2013. DNA barcoding freshwater Rotifera of Mexico: evidence of cryptic speciation in common rotifers. Mol Ecol Resour 13: 1097–1107. [Google Scholar]
- García-Moralez AE, Domínguez-Domínguez O. 2020. Cryptic species within the rotifer Lecane bulla (Rotifera: Monogononta: Lecanidae) from North America based on molecular species delimitation. Rev Mex Biodivers 91: e913116. [Google Scholar]
- García-Roger E, Carmona MJ, Serra M. 2006. Patterns in rotifer diapausing egg banks: density and viability. J Exp Mar Biol Ecol 2: 198–210. [Google Scholar]
- Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. 2002. Effects of size and temperature on developmental time. Nature 417: 70–73. [CrossRef] [PubMed] [Google Scholar]
- Hummon WD, Bevelhymer DP. 1979. Life table demography of the rotifer Lecane tenuiseta under culture conditions, and various age distribution. Hydrobiologia 70: 25–28. [Google Scholar]
- Kordbacheh A, WallaceI RL, Walsh EJ. 2018. Evidence supporting cryptic species within two sessile microinvertebrates, Limnias melicerta and L. ceratophylli (Rotifera, Gnesiotrocha). PLoS ONE 13. [Google Scholar]
- Koste W. 2000. Study of the Rotatoria-Fauna of the Littoral of the Rio Branco, South of Boa Vista, Northern Brazil. Int Rev Hydrobiol 85: 433469. [Google Scholar]
- Krebs CJ. 1985. Ecología: Estudio de la distribución y la abundancia, 2nd edn. México City: Editorial Harla, 753 p. [Google Scholar]
- Leasi F, Tang CQ, De Smet WH, Fontaneto D. 2013. Cryptic diversity with wide salinity tolerance in the putative euryhaline Testudinella clypeata (Rotifera, Monogononta). Zool J Linn Soc 168: 17–28. [Google Scholar]
- Leliaert F, Verbruggen H, Vanormelingen P, Steen F, López-Bautista J, Zuccarello G, Clerck O. 2014. DNA-based species delimitation in algae. Br Phycol Soc 49: 179–196. [Google Scholar]
- Ma Q, Xi YL, Zhang JY, Wen XL, Xiang XL. 2010. Differences in life table demography among eight geographic populations of Brachionus calyciflorus (Rotifera) from China. Limnologica 40: 16–22. [Google Scholar]
- Michaloudi E, Papakostas S, Stamou G, Nedela V, Tihlarikova E, Zhang W, Steven A, Declerck J. 2018. Reverse taxonomy applied to the Brachionus calyciflorus cryptic species complex: Morphometric analysis confirms species delimitations revealed by molecular phylogenetic analysis and allows the (re)description of four species. PLoS ONE 13. [Google Scholar]
- Mills S, Alcántara-Rodríguez JA, Ciros-Pérez J, Gómez A, Hagiwara A, Galindo KH, Jersabek CD, Malekzadeh-Viayeh R, Leasi F, Lee JS, et al. 2017. Fifteen species in one: deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 796: 39–58. [Google Scholar]
- Moreno E, Conde-Porcuna JM, Gómez A. 2017. Barcoding rotifer biodiversity in Mediterranean ponds using diapausing egg banks. Ecol Evol 7: 4855–4867. [Google Scholar]
- Nichols HW. 1973. Growth media-freshwater. In: Stein JR (ed.), Handbook of Phycological Methods. Cambridge: Cambridge University Press. [Google Scholar]
- Obertegger U, Flaim G, Fontaneto D. 2014. Cryptic diversity within the rotifer Polyarthra dolichoptera along an altitudinal gradient. Freshw Biol 59: 2413–2427. [Google Scholar]
- Ortells R, Snell TW, Gómez A, Serra M. 2000. Patterns of genetic differentiation in resting egg banks of a rotifer species complex in Spain. Arch Hydrobiol 149: 529–551. [Google Scholar]
- Perez-Legaspi IA, Rico-Martínez R. 1998. Effect of temperature and food concentration in two species of littoral rotifers. Hydrobiologia 387: 341–348 [Google Scholar]
- Sarma SSS, Jiménez-Santos MA, Nandini S, Wallace RL. 2017. Demography of the sessile rotifers, Limnias ceratophylli and Limnias melicerta (Rotifera: Gnesiotrocha), in relation to food (Chlorella vulgaris Beijerinck, 1890) density. Hydrobiologia 796: 181–189. [Google Scholar]
- Saucedo-Ríos S, Santos-Medrano GE, Rico-Martínez R. 2017. Life table analysis reveals variation in thermal tolerance among three species of the Lecane genus (Rotifera: Monogononta). Ann Limnol 53: 253–259. [Google Scholar]
- Segers H. 1995. Rotifera Vol. 2 The Lecanidae (Monogononta). The Netherlands: The Hague. SPB Academic Publishing, pp. 226. [Google Scholar]
- Segers H. 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595: 49–59. [Google Scholar]
- Segers H, Rico-Martínez R. 2000. The male of Lecane bulla (Gosse, 1851): new support for the synonymy of Lecane Nitzsch, Monostyla Ehrenberg and Hemimonostyla Bartos. J Nat Hist 34: 679–683. [Google Scholar]
- Serranía-Soto C, Sarma SSS, Nandini S. 2011. Studies on comparative population growth of some species of the rotifer Lecane (Rotifera). J Environ Biol 32: 523–527. [PubMed] [Google Scholar]
- Serranía-Soto CR. 2003. Some taxonomical aspects of Rotifera from central Mexico. Sci Nat 6: 53–61. [Google Scholar]
- Stelzer CP. 2017. Extremely short diapause in rotifers and its fitness consequences. Hydrobiologia 796: 255–264. [Google Scholar]
- Sudzuki M. 1999. An approach to the identification of the common rotifers. Tokyo: Sanseido, 151 pp. [Google Scholar]
- Walsh EJ, Schroder T, Wallace RL, Rico-Martínez R. 2009. Cryptic speciation in Lecane bulla (Monogononta: Rotifera) in Chihuahuan Desert waters. Verh Internat Verein Limnol 30: 1046–1050. [Google Scholar]
- Xi YL, Xu DD, Ma J, Ge YL, Wen XL. 2013. Differences in life table parameters between Keratella tropica and Keratella vaga (Rotatoria) from subtropical shallow lakes. J Freshw Ecol 28: 539–554. [Google Scholar]
- Xu-Wang Y, Bing-Bing T, Yan-Chun Z, Xiao-Chun, Wei L. 2016. Development time of male and female rotifers with sexual size dimorphism. Hydrobiologia 767: 27–35. [Google Scholar]
Cite this article as: Arroyo-Castro JL, Rico-Martínez R, Alvarado-Flores J. 2021. Study of morphotypes and life history of six clones of Lecane bulla (Gosse, 1851) from Quintana Roo, Mexico. Ann. Limnol. - Int. J. Lim. 57: 9
All Tables
Environmental data of sampled localities: number of strains; temperature (°C), conductivity (μs/cm), and pH.
Demographic parameters reported for Lecane bulla: lifespan (L; days), instantaneous growth rate of the population (r), generation time (T; days), net reproductive rate (R0) and number of male offspring total (male).
All Figures
 |
Fig. 1 Survivorship (diamond) and reproductive value (square) of Lecane bulla from six sites in Quintana Roo. COR: Corchalito, PC: Parque del cenote, SCR: El secreto; PL: Punta Laguna, MYL: Muyil, CHM: Chemuyil. X axis days.With a total of 18 replicates. |
| In the text | |
 |
Fig. 2 Relationship between the demographic parameters (LN) and the body size (length, µm) of the six localities of Lecane bulla of Quintana Roo. N = 20. |
| In the text | |
 |
Fig. 3 Relationship between the egg volumen (10 × 4 μm3) and the body size (length, µm) of the six localities of Lecane bulla of Quintana Roo. N = 20. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


